Stx5-Mediated ER-Golgi Transport in Mammals and Yeast
- PMID: 31357511
- PMCID: PMC6721632
- DOI: 10.3390/cells8080780
Stx5-Mediated ER-Golgi Transport in Mammals and Yeast
Abstract
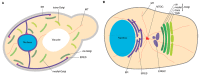
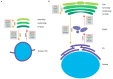
The soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) syntaxin 5 (Stx5) in mammals and its ortholog Sed5p in Saccharomyces cerevisiae mediate anterograde and retrograde endoplasmic reticulum (ER)-Golgi trafficking. Stx5 and Sed5p are structurally highly conserved and are both regulated by interactions with other ER-Golgi SNARE proteins, the Sec1/Munc18-like protein Scfd1/Sly1p and the membrane tethering complexes COG, p115, and GM130. Despite these similarities, yeast Sed5p and mammalian Stx5 are differently recruited to COPII-coated vesicles, and Stx5 interacts with the microtubular cytoskeleton, whereas Sed5p does not. In this review, we argue that these different Stx5 interactions contribute to structural differences in ER-Golgi transport between mammalian and yeast cells. Insight into the function of Stx5 is important given its essential role in the secretory pathway of eukaryotic cells and its involvement in infections and neurodegenerative diseases.
Keywords: Golgi apparatus; endoplasmic reticulum; membrane trafficking; secretory pathway; syntaxin 5.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Sly1 protein bound to Golgi syntaxin Sed5p allows assembly and contributes to specificity of SNARE fusion complexes.J Cell Biol. 2002 May 13;157(4):645-55. doi: 10.1083/jcb.200202006. Epub 2002 May 6. J Cell Biol. 2002. PMID: 11994317 Free PMC article.
-
Cysteine-disulfide cross-linking to monitor SNARE complex assembly during endoplasmic reticulum-Golgi transport.J Biol Chem. 2006 Jan 27;281(4):2281-8. doi: 10.1074/jbc.M511695200. Epub 2005 Nov 21. J Biol Chem. 2006. PMID: 16303754
-
Stx5 is a novel interactor of VLDL-R to affect its intracellular trafficking and processing.Exp Cell Res. 2013 Aug 1;319(13):1956-1972. doi: 10.1016/j.yexcr.2013.05.010. Epub 2013 May 20. Exp Cell Res. 2013. PMID: 23701949
-
SNAREs and membrane fusion in the Golgi apparatus.Biochim Biophys Acta. 1998 Aug 14;1404(1-2):9-31. doi: 10.1016/s0167-4889(98)00044-5. Biochim Biophys Acta. 1998. PMID: 9714710 Review.
-
The role of Sec1p-related proteins in vesicle trafficking in the nerve terminal.J Neurosci Res. 1996 Jul 15;45(2):89-95. doi: 10.1002/(SICI)1097-4547(19960715)45:2<89::AID-JNR1>3.0.CO;2-B. J Neurosci Res. 1996. PMID: 8843026 Review.
Cited by
-
Golgi-associated Rab GTPases implicated in autophagy.Cell Biosci. 2021 Feb 8;11(1):35. doi: 10.1186/s13578-021-00543-2. Cell Biosci. 2021. PMID: 33557950 Free PMC article. Review.
-
An Update on the Key Factors Required for Plant Golgi Structure Maintenance.Front Plant Sci. 2022 Jun 28;13:933283. doi: 10.3389/fpls.2022.933283. eCollection 2022. Front Plant Sci. 2022. PMID: 35837464 Free PMC article. Review.
-
Golgi clustering by the deficiency of COPI-SNARE in Drosophila photoreceptors.Front Cell Dev Biol. 2024 Sep 4;12:1442198. doi: 10.3389/fcell.2024.1442198. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39296936 Free PMC article.
-
Golgi Apparatus: A Potential Therapeutic Target for Autophagy-Associated Neurological Diseases.Front Cell Dev Biol. 2020 Sep 9;8:564975. doi: 10.3389/fcell.2020.564975. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33015059 Free PMC article. Review.
-
Dispatch and delivery at the ER-Golgi interface: how endothelial cells tune their hemostatic response.FEBS J. 2022 Nov;289(22):6863-6870. doi: 10.1111/febs.16421. Epub 2022 Mar 10. FEBS J. 2022. PMID: 35246944 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

