Self-DNA at the Epicenter of SLE: Immunogenic Forms, Regulation, and Effects
- PMID: 31354738
- PMCID: PMC6637313
- DOI: 10.3389/fimmu.2019.01601
Self-DNA at the Epicenter of SLE: Immunogenic Forms, Regulation, and Effects
Abstract
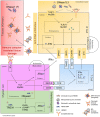
Self-reactive B cells generated through V(D)J recombination in the bone marrow or through accrual of random mutations in secondary lymphoid tissues are mostly purged or edited to prevent autoimmunity. Yet, 10-20% of all mature naïve B cells in healthy individuals have self-reactive B cell receptors (BCRs). In patients with serologically active systemic lupus erythematosus (SLE) the percentage increases up to 50%, with significant self-DNA reactivity that correlates with disease severity. Endogenous or self-DNA has emerged as a potent antigen in several autoimmune disorders, particularly in SLE. However, the mechanism(s) regulating or preventing anti-DNA antibody production remain elusive. It is likely that in healthy subjects, DNA-reactive B cells avoid activation due to the unavailability of endogenous DNA, which is efficiently degraded through efferocytosis and various DNA-processing proteins. Genetic defects, physiological, and/or pathological conditions can override these protective checkpoints, leading to autoimmunity. Plausibly, increased availability of immunogenic self-DNA may be the key initiating event in the loss of tolerance of otherwise quiescent DNA-reactive B cells. Indeed, mutations impairing apoptotic cell clearance pathways and nucleic acid metabolism-associated genes like DNases, RNases, and their sensors are known to cause autoimmune disorders including SLE. Here we review the literature supporting the idea that increased availability of DNA as an immunogen or adjuvant, or both, may cause the production of pathogenic anti-DNA antibodies and subsequent manifestations of clinical disease such as SLE. We discuss the main cellular players involved in anti-DNA responses; the physical forms and sources of immunogenic DNA in autoimmunity; the DNA-protein complexes that render DNA immunogenic; the regulation of DNA availability by intracellular and extracellular DNases and the autoimmune pathologies associated with their dysfunction; the cytosolic and endosomal sensors of immunogenic DNA; and the cytokines such as interferons that drive auto-inflammatory and autoimmune pathways leading to clinical disease. We propose that prevention of DNA availability by aiding extracellular DNase activity could be a viable therapeutic modality in controlling SLE.
Keywords: DNases; autoantibodies; interferons; systemic lupus erythematosus; toll-like receptors.
Figures
Similar articles
-
Plasmacytoid Dendritic Cells and Type I Interferon Promote Extrafollicular B Cell Responses to Extracellular Self-DNA.Immunity. 2020 Jun 16;52(6):1022-1038.e7. doi: 10.1016/j.immuni.2020.04.015. Epub 2020 May 25. Immunity. 2020. PMID: 32454024 Free PMC article.
-
Self-dsDNA in the pathogenesis of systemic lupus erythematosus.Clin Exp Immunol. 2018 Jan;191(1):1-10. doi: 10.1111/cei.13041. Epub 2017 Sep 15. Clin Exp Immunol. 2018. PMID: 28836661 Free PMC article. Review.
-
The B cell response to both protein and nucleic acid antigens displayed on apoptotic cells are dependent on endosomal pattern recognition receptors.J Autoimmun. 2021 Feb;117:102582. doi: 10.1016/j.jaut.2020.102582. Epub 2020 Dec 6. J Autoimmun. 2021. PMID: 33296829
-
Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus.Sci Transl Med. 2011 Mar 9;3(73):73ra19. doi: 10.1126/scitranslmed.3001180. Sci Transl Med. 2011. PMID: 21389263 Free PMC article.
-
The role of innate immunity in the induction of autoimmunity.Autoimmun Rev. 2008 Oct;8(1):69-72. doi: 10.1016/j.autrev.2008.07.028. Epub 2008 Aug 15. Autoimmun Rev. 2008. PMID: 18708168 Review.
Cited by
-
Self-DNA Sensing by cGAS-STING and TLR9 in Autoimmunity: Is the Cytoskeleton in Control?Front Immunol. 2021 May 18;12:657344. doi: 10.3389/fimmu.2021.657344. eCollection 2021. Front Immunol. 2021. PMID: 34084165 Free PMC article. Review.
-
Innate and adaptive immune abnormalities underlying autoimmune diseases: the genetic connections.Sci China Life Sci. 2023 Jul;66(7):1482-1517. doi: 10.1007/s11427-021-2187-3. Epub 2023 Feb 3. Sci China Life Sci. 2023. PMID: 36738430 Free PMC article. Review.
-
Origin and significance of the human DNase repertoire.Sci Rep. 2022 Jun 20;12(1):10364. doi: 10.1038/s41598-022-14133-w. Sci Rep. 2022. PMID: 35725583 Free PMC article.
-
The Role and Mechanism of Metformin in Inflammatory Diseases.J Inflamm Res. 2023 Nov 23;16:5545-5564. doi: 10.2147/JIR.S436147. eCollection 2023. J Inflamm Res. 2023. PMID: 38026260 Free PMC article. Review.
-
Type I Interferons in Autoimmunity.J Invest Dermatol. 2022 Mar;142(3 Pt B):793-803. doi: 10.1016/j.jid.2021.11.031. Epub 2022 Jan 10. J Invest Dermatol. 2022. PMID: 35016780 Free PMC article. Review.
References
-
- Ter Borg EJ, Horst G, Hummel EJ, Limburg PC, Kallenberg CG. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus. A long-term, prospective study. Arthritis Rheum. (1990) 33:634–43. 10.1002/art.1780330505 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

