High-Density Lipoprotein in Lupus: Disease Biomarkers and Potential Therapeutic Strategy
- PMID: 31350818
- PMCID: PMC6935404
- DOI: 10.1002/art.41059
High-Density Lipoprotein in Lupus: Disease Biomarkers and Potential Therapeutic Strategy
Abstract
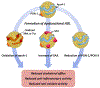
Systemic lupus erythematosus (SLE) patients exhibit accelerated development of atherosclerosis and increased incidents of cardiovascular disease (CVD) that cannot be explained by traditional risk factors alone. Accumulating evidence suggests that reduced levels of high-density lipoproteins (HDLs), along with altered HDL composition and function, may contribute to the accelerated atherosclerosis in SLE patients. Normally, HDLs play various atheroprotective roles through facilitating cholesterol efflux, inhibiting vascular inflammation, and scavenging oxidative species. However, systemic inflammation, oxidative stress, and autoimmunity in SLE patients induce changes in HDL size distribution and proteomic and lipidomic signatures. These compositional changes in HDLs result in the formation of proinflammatory, dysfunctional HDL. These lupus-altered HDLs have impaired antiatherogenic function with reduced cholesterol efflux capacities, impaired antioxidation abilities, and diminished antiinflammatory properties. In fact, dysfunctional HDL may promote atherogenesis by inducing inflammation. Thus, dysfunctional HDLs could be an important biomarker of accelerated atherosclerosis in lupus. Additionally, HDL-targeted therapies, especially infusion of reconstituted HDLs, may serve as a potential therapeutic intervention for SLE patients with CVD.
© 2019, American College of Rheumatology.
Conflict of interest statement
There are no financial conflicts of interest to disclose.
Figures

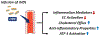
Comment in
-
Anti-Apolipoprotein A-I in Systemic Lupus Erythematosus: Comment on the Article by Kim et al.Arthritis Rheumatol. 2020 Jul;72(7):1233-1234. doi: 10.1002/art.41235. Epub 2020 May 31. Arthritis Rheumatol. 2020. PMID: 32103622 No abstract available.
-
Reply.Arthritis Rheumatol. 2020 Jul;72(7):1234-1236. doi: 10.1002/art.41237. Epub 2020 May 28. Arthritis Rheumatol. 2020. PMID: 32103638 No abstract available.
Similar articles
-
Altered lipoprotein metabolism in chronic inflammatory states: proinflammatory high-density lipoprotein and accelerated atherosclerosis in systemic lupus erythematosus and rheumatoid arthritis.Arthritis Res Ther. 2008;10(4):213. doi: 10.1186/ar2471. Epub 2008 Aug 29. Arthritis Res Ther. 2008. PMID: 18828865 Free PMC article. Review.
-
Role of dyslipidemia in accelerating inflammation, autoimmunity, and atherosclerosis in systemic lupus erythematosus and other autoimmune diseases.Discov Med. 2020 Jul-Aug;30(159):49-56. Discov Med. 2020. PMID: 33357362 Review.
-
Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis.Arthritis Rheum. 2006 Aug;54(8):2541-9. doi: 10.1002/art.21976. Arthritis Rheum. 2006. PMID: 16868975
-
High-density lipoprotein functionality in systemic lupus erythematosus.Semin Arthritis Rheum. 2020 Aug;50(4):769-775. doi: 10.1016/j.semarthrit.2020.05.011. Epub 2020 May 25. Semin Arthritis Rheum. 2020. PMID: 32531506 Review.
-
Dyslipidaemia and lipoprotein pattern in systemic lupus erythematosus (SLE) and SLE-related cardiovascular disease.Scand J Rheumatol. 2009 May-Jun;38(3):184-9. doi: 10.1080/03009740802541470. Scand J Rheumatol. 2009. PMID: 19165647
Cited by
-
TNF Inhibitors Exert a "Hidden" Beneficial Effect in the Cardiovascular Lipoprotein Profile of RA Patients.Biologics. 2022 Oct 17;16:187-197. doi: 10.2147/BTT.S364191. eCollection 2022. Biologics. 2022. PMID: 36281333 Free PMC article.
-
Immune mechanisms associated with cardiovascular disease in systemic lupus erythematosus: A path to potential biomarkers.Front Immunol. 2022 Nov 7;13:974826. doi: 10.3389/fimmu.2022.974826. eCollection 2022. Front Immunol. 2022. PMID: 36420265 Free PMC article. Review.
-
Enhancing Wound Healing and Anti-Inflammatory Effects by Combination of CIGB-258 and Apolipoprotein A-I against Carboxymethyllysine Toxicity in Zebrafish: Insights into Structural Stabilization and Antioxidant Properties.Antioxidants (Basel). 2024 Aug 28;13(9):1049. doi: 10.3390/antiox13091049. Antioxidants (Basel). 2024. PMID: 39334708 Free PMC article.
-
HDL in Immune-Inflammatory Responses: Implications beyond Cardiovascular Diseases.Cells. 2021 Apr 29;10(5):1061. doi: 10.3390/cells10051061. Cells. 2021. PMID: 33947039 Free PMC article. Review.
-
Association of serum lipids with inflammatory bowel disease: a systematic review and meta-analysis.Front Med (Lausanne). 2023 Aug 24;10:1198988. doi: 10.3389/fmed.2023.1198988. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37692785 Free PMC article. Review.
References
-
- Urowitz MB, Gladman DD, Tom BDM, Ibañez D & Farewell VT Changing patterns in mortality and disease outcomes for patients with systemic lupus erythematosus. J. Rheumatol 35, 2152–8 (2008). - PubMed
-
- Bernatsky S et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 54, 2550–2557 (2006). - PubMed
-
- Agarwal S, Elliott JR & Manzi S Atherosclerosis risk factors in systemic lupus erythematosus. Curr. Rheumatol. Rep 11, 241–247 (2009). - PubMed
-
- Lerang K, Gilboe I-M, Steinar Thelle D & Gran JT Mortality and years of potential life loss in systemic lupus erythematosus: a population-based cohort study. Lupus 23, 1546–1552 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

