N-cadherin overexpression enhances the reparative potency of human-induced pluripotent stem cell-derived cardiac myocytes in infarcted mouse hearts
- PMID: 31350544
- PMCID: PMC8204485
- DOI: 10.1093/cvr/cvz179
N-cadherin overexpression enhances the reparative potency of human-induced pluripotent stem cell-derived cardiac myocytes in infarcted mouse hearts
Abstract
Aims: In regenerative medicine, cellular cardiomyoplasty is one of the promising options for treating myocardial infarction (MI); however, the efficacy of such treatment has shown to be limited due to poor survival and/or functional integration of implanted cells. Within the heart, the adhesion between cardiac myocytes (CMs) is mediated by N-cadherin (CDH2) and is critical for the heart to function as an electromechanical syncytium. In this study, we have investigated whether the reparative potency of human-induced pluripotent stem cell-derived cardiac myocytes (hiPSC-CMs) can be enhanced through CDH2 overexpression.
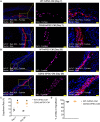
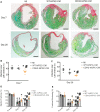
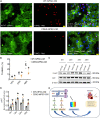
Methods and results: CDH2-hiPSC-CMs and control wild-type (WT)-hiPSC-CMs were cultured in myogenic differentiation medium for 28 days. Using a mouse MI model, the cell survival/engraftment rate, infarct size, and cardiac functions were evaluated post-MI, at Day 7 or Day 28. In vitro, conduction velocities were significantly greater in CDH2-hiPSC-CMs than in WT-hiPSC-CMs. While, in vivo, measurements of cardiac functions: left ventricular (LV) ejection fraction, reduction in infarct size, and the cell engraftment rate were significantly higher in CDH2-hiPSC-CMs treated MI group than in WT-hiPSC-CMs treated MI group. Mechanistically, paracrine activation of ERK signal transduction pathway by CDH2-hiPSC-CMs, significantly induced neo-vasculogenesis, resulting in a higher survival of implanted cells.
Conclusion: Collectively, these data suggest that CDH2 overexpression enhances not only the survival/engraftment of cultured CDH2-hiPSC-CMs, but also the functional integration of these cells, consequently, the augmentation of the reparative properties of implanted CDH2-hiPSC-CMs in the failing hearts.
Keywords: Cardiac myocytes; Cardiac regeneration; Electro-mechanical syncytium; Human-induced pluripotent stem cells; Myocardial infarction; N-cadherin.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2019. For permissions, please email: journals.permissions@oup.com.
Figures

Comment in
-
Improving the engraftment and integration of cell transplantation for cardiac regeneration.Cardiovasc Res. 2020 Mar 1;116(3):473-475. doi: 10.1093/cvr/cvz237. Cardiovasc Res. 2020. PMID: 31504255 Free PMC article. No abstract available.
Similar articles
-
Angiopoietin-1 enhanced myocyte mitosis, engraftment, and the reparability of hiPSC-CMs for treatment of myocardial infarction.Cardiovasc Res. 2021 May 25;117(6):1578-1591. doi: 10.1093/cvr/cvaa215. Cardiovasc Res. 2021. PMID: 32666104
-
Thymosin β4 increases cardiac cell proliferation, cell engraftment, and the reparative potency of human induced-pluripotent stem cell-derived cardiomyocytes in a porcine model of acute myocardial infarction.Theranostics. 2021 Jun 26;11(16):7879-7895. doi: 10.7150/thno.56757. eCollection 2021. Theranostics. 2021. PMID: 34335970 Free PMC article.
-
Y-27632 preconditioning enhances transplantation of human-induced pluripotent stem cell-derived cardiomyocytes in myocardial infarction mice.Cardiovasc Res. 2019 Feb 1;115(2):343-356. doi: 10.1093/cvr/cvy207. Cardiovasc Res. 2019. PMID: 30107391 Free PMC article.
-
HiPS-Cardiac Trilineage Cell Generation and Transplantation: a Novel Therapy for Myocardial Infarction.J Cardiovasc Transl Res. 2020 Feb;13(1):110-119. doi: 10.1007/s12265-019-09891-4. Epub 2019 May 31. J Cardiovasc Transl Res. 2020. PMID: 31152358 Review.
-
Embryonic template-based generation and purification of pluripotent stem cell-derived cardiomyocytes for heart repair.J Cardiovasc Transl Res. 2012 Oct;5(5):566-80. doi: 10.1007/s12265-012-9391-6. Epub 2012 Jul 18. J Cardiovasc Transl Res. 2012. PMID: 22806916 Review.
Cited by
-
Challenges and perspectives of heart repair with pluripotent stem cell-derived cardiomyocytes.Nat Cardiovasc Res. 2024 May;3(5):515-524. doi: 10.1038/s44161-024-00472-6. Epub 2024 May 14. Nat Cardiovasc Res. 2024. PMID: 39195938 Review.
-
Doxycycline-Mediated Control of Cyclin D2 Overexpression in Human-Induced Pluripotent Stem Cells.Int J Mol Sci. 2024 Aug 9;25(16):8714. doi: 10.3390/ijms25168714. Int J Mol Sci. 2024. PMID: 39201401 Free PMC article.
-
Chitosan-Based Scaffolds for the Treatment of Myocardial Infarction: A Systematic Review.Molecules. 2023 Feb 17;28(4):1920. doi: 10.3390/molecules28041920. Molecules. 2023. PMID: 36838907 Free PMC article. Review.
-
Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes, in Contrast to Adipose Tissue-Derived Stromal Cells, Efficiently Improve Heart Function in Murine Model of Myocardial Infarction.Biomedicines. 2020 Dec 7;8(12):578. doi: 10.3390/biomedicines8120578. Biomedicines. 2020. PMID: 33297443 Free PMC article.
-
Engineering of thick human functional myocardium via static stretching and electrical stimulation.iScience. 2022 Feb 4;25(3):103824. doi: 10.1016/j.isci.2022.103824. eCollection 2022 Mar 18. iScience. 2022. PMID: 35243219 Free PMC article.
References
-
- Soonpaa MH, Field LJ. Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ Res 1998;83:15–26. - PubMed
-
- Fuchs E, Segre JA. Stem cells: a new lease on life. Cell 2000;100:143–155. - PubMed
-
- Ye L, Chang YH, Xiong Q, Zhang P, Zhang L, Somasundaram P, Lepley M, Swingen C, Su L, Wendel JS, Guo J, Jang A, Rosenbush D, Greder L, Dutton JR, Zhang J, Kamp TJ, Kaufman DS, Ge Y, Zhang J. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell 2014;15:750–761. - PMC - PubMed
-
- Huang P, Tian X, Li Q, Yang Y. New strategies for improving stem cell therapy in ischemic heart disease. Heart Fail Rev 2016;21:737–752. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

