Osteoblasts grown on microroughened titanium surfaces regulate angiogenic growth factor production through specific integrin receptors
- PMID: 31349056
- PMCID: PMC7250132
- DOI: 10.1016/j.actbio.2019.07.036
Osteoblasts grown on microroughened titanium surfaces regulate angiogenic growth factor production through specific integrin receptors
Abstract
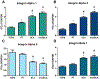
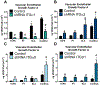
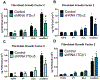
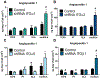
Cellular attachment and response to biomaterials are mediated by integrin receptor binding to extracellular matrix proteins adsorbed onto the material surface. Osteoblasts interact with their substrates via several integrin complexes including fibronectin-binding α5β1 and collagen-binding α1β1 and α2β1. Knockdown of α2 or β1 integrin subunits inhibits the production of factors that promote an osteogenic microenvironment, including osteocalcin, osteoprotegerin, and TGFβ1. Osteoblasts also secrete several angiogenic growth factors such as VEGF-A (VEGF165), FGF-2, and angiopoietin 1, which are regulated by titanium surface topography and surface energy. Here, we examined whether signaling through integrin receptor complexes regulates production and secretion of angiogenic factors during osteoblast differentiation on microtextured Ti surfaces. To do this, integrin subunits α1, α2, α5, and β1 were stably silenced in MG63 osteoblast-like cells cultured on grit-blasted/acid-etched hydrophobic Ti (SLA) or on hydrophilic SLA (modSLA). VEGF-A production increased in response to Ti surface topography and energy in integrin α2, α5, and β1 silenced cells but decreased in α1-silenced cells. FGF-2 decreased on modSLA substrates in both α1 and α2-silenced cells but was unchanged in response to silencing of either α5 or β1. In integrin α1, α2, and β1-silenced cells, Ang-1 increased on modSLA but α5-silencing did not affect Ang-1 production during surface mediated differentiation. These results suggest that signaling through specific integrin receptor complexes during osteoblast differentiation on microstructured Ti substrates, regulates the production of angiogenic factors by those cells, and this is differentially regulated by surface hydrophilicity. STATEMENT OF SIGNIFICANCE: Successful implantation of synthetic biomaterials into bone depends on the biological process known as osseointegration. Osseointegration is a highly regulated communication of cells that orchestrates the migration of progenitor cells towards the implant site and promotes the deposition and mineralization of extracellular matrix proteins within the implant microenvironment, to tightly join the implant to native bone. In this process, angiogenesis functions as the initiation site of progenitor cell migration and is necessary for matrix deposition by providing the necessary nutrients for bone formation. In the present study, we show a novel regulation of specific angiogenic growth factors by integrin receptor complexes. This research is important to develop biomaterials that promote and maintain osseointegration through proper vascularization and prevent implant failure in patients lacking sufficient angiogenesis.
Keywords: Angiogenesis; Differentiation; Integrins; Osteoblast; Osteocalcin (OCN); Surface; Titanium.
Copyright © 2019 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Integrin alpha2beta1 plays a critical role in osteoblast response to micron-scale surface structure and surface energy of titanium substrates.Proc Natl Acad Sci U S A. 2008 Oct 14;105(41):15767-72. doi: 10.1073/pnas.0805420105. Epub 2008 Oct 8. Proc Natl Acad Sci U S A. 2008. PMID: 18843104 Free PMC article.
-
Regulation of angiogenesis during osseointegration by titanium surface microstructure and energy.Biomaterials. 2010 Jun;31(18):4909-17. doi: 10.1016/j.biomaterials.2010.02.071. Epub 2010 Mar 30. Biomaterials. 2010. PMID: 20356623 Free PMC article.
-
VEGF-A regulates angiogenesis during osseointegration of Ti implants via paracrine/autocrine regulation of osteoblast response to hierarchical microstructure of the surface.J Biomed Mater Res A. 2019 Feb;107(2):423-433. doi: 10.1002/jbm.a.36559. Epub 2018 Nov 21. J Biomed Mater Res A. 2019. PMID: 30461195 Free PMC article.
-
Influence of substratum surface chemistry/energy and topography on the human fetal osteoblastic cell line hFOB 1.19: Phenotypic and genotypic responses observed in vitro.Biomaterials. 2007 Nov;28(31):4535-50. doi: 10.1016/j.biomaterials.2007.06.016. Epub 2007 Jul 20. Biomaterials. 2007. PMID: 17644175 Free PMC article. Review.
-
The Roles of Integrin α5β1 in Human Cancer.Onco Targets Ther. 2020 Dec 31;13:13329-13344. doi: 10.2147/OTT.S273803. eCollection 2020. Onco Targets Ther. 2020. PMID: 33408483 Free PMC article. Review.
Cited by
-
Analysis of disordered abrasive scratches on titanium surfaces and their impact on nuclear translocation of yes-associated protein.Sci Rep. 2022 Dec 15;12(1):21705. doi: 10.1038/s41598-022-26203-0. Sci Rep. 2022. PMID: 36522392 Free PMC article.
-
NanoZnO-modified titanium implants for enhanced anti-bacterial activity, osteogenesis and corrosion resistance.J Nanobiotechnology. 2021 Oct 30;19(1):353. doi: 10.1186/s12951-021-01099-6. J Nanobiotechnology. 2021. PMID: 34717648 Free PMC article. Review.
-
Enhanced VEGF/VEGF-R and RUNX2 Expression in Human Periodontal Ligament Stem Cells Cultured on Sandblasted/Etched Titanium Disk.Front Cell Dev Biol. 2020 May 14;8:315. doi: 10.3389/fcell.2020.00315. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32478069 Free PMC article.
-
Hierarchical Micro-Nano Topography Promotes Cell Adhesion and Osteogenic Differentiation via Integrin α2-PI3K-AKT Signaling Axis.Front Bioeng Biotechnol. 2020 May 19;8:463. doi: 10.3389/fbioe.2020.00463. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32509748 Free PMC article.
-
Osseointegration of Titanium Implants in a Botox-Induced Muscle Paralysis Rat Model Is Sensitive to Surface Topography and Semaphorin 3A Treatment.Biomimetics (Basel). 2023 Feb 25;8(1):93. doi: 10.3390/biomimetics8010093. Biomimetics (Basel). 2023. PMID: 36975323 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

