Gene loci associated with insulin secretion in islets from non-diabetic mice
- PMID: 31343992
- PMCID: PMC6763251
- DOI: 10.1172/JCI129143
Gene loci associated with insulin secretion in islets from non-diabetic mice
Abstract
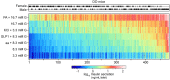
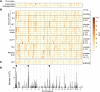
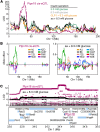
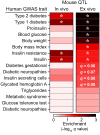
Genetic susceptibility to type 2 diabetes is primarily due to β-cell dysfunction. However, a genetic study to directly interrogate β-cell function ex vivo has never been previously performed. We isolated 233,447 islets from 483 Diversity Outbred (DO) mice maintained on a Western-style diet, and measured insulin secretion in response to a variety of secretagogues. Insulin secretion from DO islets ranged >1,000-fold even though none of the mice were diabetic. The insulin secretory response to each secretagogue had a unique genetic architecture; some of the loci were specific for one condition, whereas others overlapped. Human loci that are syntenic to many of the insulin secretion QTL from mouse are associated with diabetes-related SNPs in human genome-wide association studies. We report on three genes, Ptpn18, Hunk and Zfp148, where the phenotype predictions from the genetic screen were fulfilled in our studies of transgenic mouse models. These three genes encode a non-receptor type protein tyrosine phosphatase, a serine/threonine protein kinase, and a Krϋppel-type zinc-finger transcription factor, respectively. Our results demonstrate that genetic variation in insulin secretion that can lead to type 2 diabetes is discoverable in non-diabetic individuals.
Keywords: Cell Biology; Diabetes; Genetics; Glucose metabolism; Mouse models.
Conflict of interest statement
Figures



Comment in
-
One small step for mice, one giant leap for GWAS?J Clin Invest. 2019 Oct 1;129(10):4083-4085. doi: 10.1172/JCI131650. J Clin Invest. 2019. PMID: 31498152 Free PMC article.
Similar articles
-
A genetic screen identifies Crat as a regulator of pancreatic beta-cell insulin secretion.Mol Metab. 2020 Jul;37:100993. doi: 10.1016/j.molmet.2020.100993. Epub 2020 Apr 13. Mol Metab. 2020. PMID: 32298772 Free PMC article.
-
Metallothionein 1 negatively regulates glucose-stimulated insulin secretion and is differentially expressed in conditions of beta cell compensation and failure in mice and humans.Diabetologia. 2019 Dec;62(12):2273-2286. doi: 10.1007/s00125-019-05008-3. Epub 2019 Oct 17. Diabetologia. 2019. PMID: 31624901
-
A common variant in TFB1M is associated with reduced insulin secretion and increased future risk of type 2 diabetes.Cell Metab. 2011 Jan 5;13(1):80-91. doi: 10.1016/j.cmet.2010.12.007. Cell Metab. 2011. PMID: 21195351
-
Transcribing β-cell mitochondria in health and disease.Mol Metab. 2017 May 31;6(9):1040-1051. doi: 10.1016/j.molmet.2017.05.014. eCollection 2017 Sep. Mol Metab. 2017. PMID: 28951827 Free PMC article. Review.
-
[Genetics of type II diabetes].Arch Mal Coeur Vaiss. 2000 Dec;93 Spec No 4:7-12. Arch Mal Coeur Vaiss. 2000. PMID: 11296466 Review. French.
Cited by
-
Regulation of protein abundance in genetically diverse mouse populations.Cell Genom. 2021 Oct 13;1(1):100003. doi: 10.1016/j.xgen.2021.100003. Epub 2021 Sep 3. Cell Genom. 2021. PMID: 36212994 Free PMC article.
-
Spontaneous Akt2 deficiency in a colony of NOD mice exhibiting early diabetes.Sci Rep. 2024 Apr 20;14(1):9100. doi: 10.1038/s41598-024-60021-w. Sci Rep. 2024. PMID: 38643275 Free PMC article.
-
Loss of ZNF148 enhances insulin secretion in human pancreatic β cells.JCI Insight. 2023 Jun 8;8(11):e157572. doi: 10.1172/jci.insight.157572. JCI Insight. 2023. PMID: 37288664 Free PMC article.
-
Proteome-wide systems genetics identifies UFMylation as a regulator of skeletal muscle function.Elife. 2022 Dec 6;11:e82951. doi: 10.7554/eLife.82951. Elife. 2022. PMID: 36472367 Free PMC article.
-
Learning a genome-wide score of human-mouse conservation at the functional genomics level.Nat Commun. 2021 May 3;12(1):2495. doi: 10.1038/s41467-021-22653-8. Nat Commun. 2021. PMID: 33941776 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

