Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated With Nivolumab
- PMID: 31343665
- PMCID: PMC6659167
- DOI: 10.1001/jamaoncol.2019.2187
Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated With Nivolumab
Abstract
Importance: Nivolumab, a monoclonal antibody that inhibits programmed cell death 1, is approved by the US Food and Drug Administration for treating advanced melanoma, renal cell carcinoma (RCC), non-small cell lung cancer (NSCLC), and other malignancies. Data on long-term survival among patients receiving nivolumab are limited.
Objectives: To analyze long-term overall survival (OS) among patients receiving nivolumab and identify clinical and laboratory measures associated with tumor regression and OS.
Design, setting, and participants: This was a secondary analysis of the phase 1 CA209-003 trial (with expansion cohorts), which was conducted at 13 US medical centers and included 270 patients with advanced melanoma, RCC, or NSCLC who received nivolumab and were enrolled between October 30, 2008, and December 28, 2011. The analyses were either specified in the original protocol or included in subsequent protocol amendments that were implemented between 2008 and 2012. Statistical analysis was performed from October 30, 2008, to November 11, 2016.
Intervention: In the CA209-003 trial, patients received nivolumab (0.1-10.0 mg/kg) every 2 weeks in 8-week cycles for up to 96 weeks, unless they developed progressive disease, achieved a complete response, experienced unacceptable toxic effects, or withdrew consent.
Main outcomes and measures: Safety and activity of nivolumab; OS was a post hoc end point with a minimum follow-up of 58.3 months.
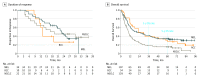
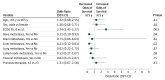
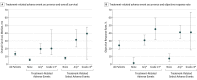
Results: Of 270 patients included in this analysis, 107 (39.6%) had melanoma (72 [67.3%] male; median age, 61 [range, 29-85] years), 34 (12.6%) had RCC (26 [76.5%] male; median age, 58 [range, 35-74] years), and 129 (47.8%) had NSCLC (79 [61.2%] male; median age, 65 [range, 38-85] years). Overall survival curves showed estimated 5-year rates of 34.2% among patients with melanoma, 27.7% among patients with RCC, and 15.6% among patients with NSCLC. In a multivariable analysis, the presence of liver (odds ratio [OR], 0.31; 95% CI, 0.12-0.83; P = .02) or bone metastases (OR, 0.31; 95% CI, 0.10-0.93; P = .04) was independently associated with reduced likelihood of survival at 5 years, whereas an Eastern Cooperative Oncology Group performance status of 0 (OR, 2.74; 95% CI, 1.43-5.27; P = .003) was independently associated with an increased likelihood of 5-year survival. Overall survival was significantly longer among patients with treatment-related AEs of any grade (median, 19.8 months; 95% CI, 13.8-26.9 months) or grade 3 or more (median, 20.3 months; 95% CI, 12.5-44.9 months) compared with those without treatment-related AEs (median, 5.8 months; 95% CI, 4.6-7.8 months) (P < .001 for both comparisons based on hazard ratios).
Conclusions and relevance: Nivolumab treatment was associated with long-term survival in a subset of heavily pretreated patients with advanced melanoma, RCC, or NSCLC. Characterizing factors associated with long-term survival may inform treatment approaches and strategies for future clinical trial development.
Trial registration: ClinicalTrials.gov identifier: NCT00730639.
Conflict of interest statement
Figures
Comment in
- doi: 10.1001/jamaoncol.2019.2186
Similar articles
-
Survival Outcomes in Patients With Previously Untreated BRAF Wild-Type Advanced Melanoma Treated With Nivolumab Therapy: Three-Year Follow-up of a Randomized Phase 3 Trial.JAMA Oncol. 2019 Feb 1;5(2):187-194. doi: 10.1001/jamaoncol.2018.4514. JAMA Oncol. 2019. PMID: 30422243 Free PMC article. Clinical Trial.
-
Nivolumab for adults with Hodgkin's lymphoma (a rapid review using the software RobotReviewer).Cochrane Database Syst Rev. 2018 Jul 12;7(7):CD012556. doi: 10.1002/14651858.CD012556.pub2. Cochrane Database Syst Rev. 2018. PMID: 30001476 Free PMC article. Review.
-
Nivolumab Plus Ipilimumab vs Nivolumab for Previously Treated Patients With Stage IV Squamous Cell Lung Cancer: The Lung-MAP S1400I Phase 3 Randomized Clinical Trial.JAMA Oncol. 2021 Sep 1;7(9):1368-1377. doi: 10.1001/jamaoncol.2021.2209. JAMA Oncol. 2021. PMID: 34264316 Free PMC article. Clinical Trial.
-
Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis.JAMA Oncol. 2016 Dec 1;2(12):1607-1616. doi: 10.1001/jamaoncol.2016.2453. JAMA Oncol. 2016. PMID: 27540850 Review.
-
Evaluation of Combination Nivolumab and Ipilimumab Immunotherapy in Patients With Advanced Biliary Tract Cancers: Subgroup Analysis of a Phase 2 Nonrandomized Clinical Trial.JAMA Oncol. 2020 Sep 1;6(9):1405-1409. doi: 10.1001/jamaoncol.2020.2814. JAMA Oncol. 2020. PMID: 32729929 Free PMC article. Clinical Trial.
Cited by
-
Why the Outcome of Anti-Tumor Immune Responses is Heterogeneous: A Novel Idea in the Context of Immunological Heterogeneity in Cancers.Bioessays. 2020 Oct;42(10):e2000024. doi: 10.1002/bies.202000024. Epub 2020 Aug 7. Bioessays. 2020. PMID: 32767371 Free PMC article.
-
Serum Metabolite Biomarkers Predictive of Response to PD-1 Blockade Therapy in Non-Small Cell Lung Cancer.Front Mol Biosci. 2021 May 21;8:678753. doi: 10.3389/fmolb.2021.678753. eCollection 2021. Front Mol Biosci. 2021. PMID: 34095230 Free PMC article.
-
An exploratory clinical study of β-glucan combined with camrelizumab and SOX chemotherapy as first-line treatment for advanced gastric adenocarcinoma.Front Immunol. 2024 Aug 26;15:1448485. doi: 10.3389/fimmu.2024.1448485. eCollection 2024. Front Immunol. 2024. PMID: 39253086 Free PMC article. Clinical Trial.
-
Three-year survival, correlates and salvage therapies in patients receiving first-line pembrolizumab for advanced Merkel cell carcinoma.J Immunother Cancer. 2021 Apr;9(4):e002478. doi: 10.1136/jitc-2021-002478. J Immunother Cancer. 2021. PMID: 33879601 Free PMC article. Clinical Trial.
-
Current challenges for assessing the long-term clinical benefit of cancer immunotherapy: a multi-stakeholder perspective.J Immunother Cancer. 2020 Jul;8(2):e000648. doi: 10.1136/jitc-2020-000648. J Immunother Cancer. 2020. PMID: 32661115 Free PMC article.
References
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

