Long-term Pilocarpine Treatment Improves Salivary Flow in Irradiated Mice
- PMID: 31341340
- PMCID: PMC6643095
- DOI: 10.1267/ahc.19006
Long-term Pilocarpine Treatment Improves Salivary Flow in Irradiated Mice
Abstract
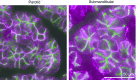
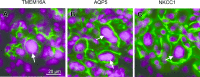
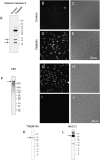
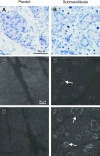
Radiation therapy for head and neck cancer frequently causes salivary gland dysfunction. Pilocarpine is a clinically approved and effective drug that induces saliva secretion, thereby keeping the oral mucosa moist and reducing discomfort in patients, but the effect is transient. We expected that this drug also has beneficial long-term effects that maintain the integrity of salivary glands by reducing, for instance, apoptosis. Here, we examined the effects of long-term pilocarpine administration in irradiated mice. The results indicated that long-term pilocarpine administration significantly improved salivary flow in irradiated mice, suggesting the potential beneficial effects of long-term administration. To elucidate the underlying mechanism, we analyzed the histology, apoptosis, and proliferation of acinar cells, and the expression of functional membrane proteins such as transmembrane member 16A, aquaporin-5, and Na-K-Cl cotransporter. Long-term pilocarpine treatment seemed to decrease irradiation-induced apoptosis, although the change was not statistically significant. The present results indicated that long-term administration of pilocarpine has beneficial effects on salivary flow in irradiated mice, and suggested that long-term administration possibly decreases apoptosis in irradiated salivary glands.
Keywords: apoptosis; pilocarpine; radiation; salivary glands; xerostomia.
Conflict of interest statement
VThe authors declare that there are no conflicts of interest.
Figures
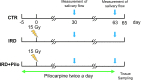
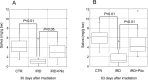

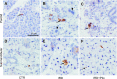

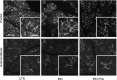


Similar articles
-
Salivation triggered by pilocarpine involves aquaporin-5 in normal rats but not in irradiated rats.Clin Exp Pharmacol Physiol. 2009 May;36(5-6):531-8. doi: 10.1111/j.1440-1681.2008.05104.x. Epub 2008 Oct 28. Clin Exp Pharmacol Physiol. 2009. PMID: 19673936
-
Epithelial disruptions, but not immune cell invasion, induced secretory dysfunction following innate immune activation in a novel model of acute salivary gland injury.J Oral Pathol Med. 2018 Feb;47(2):211-219. doi: 10.1111/jop.12663. Epub 2017 Dec 18. J Oral Pathol Med. 2018. PMID: 29160910
-
Radiation-induced hyposalivation and its treatment with oral pilocarpine.Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998 Nov;86(5):541-9. doi: 10.1016/s1079-2104(98)90343-2. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998. PMID: 9830645 Clinical Trial.
-
Oral pilocarpine: a review of its pharmacological properties and clinical potential in xerostomia.Drugs. 1995 Jan;49(1):143-55. doi: 10.2165/00003495-199549010-00010. Drugs. 1995. PMID: 7705213 Review.
-
The use of oral pilocarpine in xerostomia and Sjögren's syndrome.Semin Arthritis Rheum. 1999 Jun;28(6):360-7. doi: 10.1016/s0049-0172(99)80002-x. Semin Arthritis Rheum. 1999. PMID: 10406404 Review.
Cited by
-
Effects of polyphenols in non-centrifugal cane sugar on saliva secretion: in vitro and in vivo experiments and a randomized controlled trial.J Clin Biochem Nutr. 2023 Mar;72(2):171-182. doi: 10.3164/jcbn.22-114. Epub 2022 Dec 28. J Clin Biochem Nutr. 2023. PMID: 36936876 Free PMC article.
-
ZMYM3 May Promote Cell Proliferation in Small Cell Lung Carcinoma.Acta Histochem Cytochem. 2021 Oct 29;54(5):143-153. doi: 10.1267/ahc.21-00012. Epub 2021 Oct 2. Acta Histochem Cytochem. 2021. PMID: 34764523 Free PMC article.
-
Differential Localization and Invasion of Tumor Cells in Mouse Models of Human and Murine Leukemias.Acta Histochem Cytochem. 2020 Jun 26;53(3):43-53. doi: 10.1267/ahc.19035. Epub 2020 Apr 29. Acta Histochem Cytochem. 2020. PMID: 32624629 Free PMC article.
-
Experimental Animal Model Systems for Understanding Salivary Secretory Disorders.Int J Mol Sci. 2020 Nov 10;21(22):8423. doi: 10.3390/ijms21228423. Int J Mol Sci. 2020. PMID: 33182571 Free PMC article. Review.
-
A Review on the Role of Pilocarpine on the Management of Xerostomia and the Importance of the Topical Administration Systems Development.Pharmaceuticals (Basel). 2022 Jun 18;15(6):762. doi: 10.3390/ph15060762. Pharmaceuticals (Basel). 2022. PMID: 35745681 Free PMC article. Review.
References
-
- Bacman S., Berra A., Sterin-Borda L. and Borda E. (2001) Muscarinic acetylcholine receptor antibodies as a new marker of dry eye Sjögren syndrome. Invest. Ophthalmol. Vis. Sci. 42; 321–327. - PubMed
-
- Catalan M. A., Kondo Y., Pena-Munzenmayer G., Jaramillo Y., Liu F., Choi S., Crandall E., Borok Z., Flodby P., Shull G. E. and Melvin J. E. (2015) A fluid secretion pathway unmasked by acinar-specific Tmem16A gene ablation in the adult mouse salivary gland. Proc. Natl. Acad. Sci. U S A 112; 2263–2268. - PMC - PubMed

