Sex-Dimorphic Behavioral Alterations and Altered Neurogenesis in U12 Intron Splicing-Defective Zrsr1 Mutant Mice
- PMID: 31331069
- PMCID: PMC6678158
- DOI: 10.3390/ijms20143543
Sex-Dimorphic Behavioral Alterations and Altered Neurogenesis in U12 Intron Splicing-Defective Zrsr1 Mutant Mice
Abstract
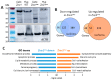
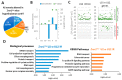
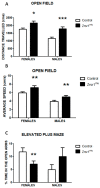
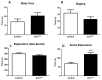
Mutant mice with respect to the splicing factor Zrsr1 present altered spermatogenesis and infertility. To investigate whether Zrsr1 is involved in the homeostatic control that the hypothalamus exerts over reproductive functions, we first analyzed both differential gene and isoform expression and alternative splicing alterations in Zrsr1 mutant (Zrsr1mu) hypothalamus; second, we analyzed the spontaneous and social behavior of Zrsr1mu mice; and third, we analyzed adult cell proliferation and survival in the Zrsr1mu hypothalamus. The Zrsr1mu hypothalamus showed altered expression of genes and isoforms related to the glutathione metabolic process, synaptonemal complex assembly, mRNA transport, and altered splicing events involving the enrichment of U12-type intron retention (IR). Furthermore, increased IR in U12-containing genes related with the prolactin, progesterone, and gonadotropin-releasing hormone (GnRH) reproductive signaling pathway was observed. This was associated with a hyperactive phenotype in both males and females, with an anxious phenotype in females, and with increased social interaction in males, instead of the classical aggressive behavior. In addition, Zrsr1mu females but not males exhibited reduced cell proliferation in both the hypothalamus and the subventricular zone. Overall, these results suggest that Zrsr1 expression and function are relevant to organization of the hypothalamic cell network controlling behavior.
Keywords: U12 introns; Zrsr1; behavior; cell proliferation; hypothalamus; neurogenesis; social interaction.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design, execution, interpretation, or writing of the study.
Figures

Similar articles
-
ZRSR1 co-operates with ZRSR2 in regulating splicing of U12-type introns in murine hematopoietic cells.Haematologica. 2022 Mar 1;107(3):680-689. doi: 10.3324/haematol.2020.260562. Haematologica. 2022. PMID: 33691379 Free PMC article.
-
Impaired Spermatogenesis, Muscle, and Erythrocyte Function in U12 Intron Splicing-Defective Zrsr1 Mutant Mice.Cell Rep. 2018 Apr 3;23(1):143-155. doi: 10.1016/j.celrep.2018.03.028. Cell Rep. 2018. PMID: 29617656
-
Minor Splicing Factors Zrsr1 and Zrsr2 Are Essential for Early Embryo Development and 2-Cell-Like Conversion.Int J Mol Sci. 2020 Jun 9;21(11):4115. doi: 10.3390/ijms21114115. Int J Mol Sci. 2020. PMID: 32527007 Free PMC article.
-
Alternative splicing of U12-type introns.Front Biosci. 2008 Jan 1;13:1681-90. doi: 10.2741/2791. Front Biosci. 2008. PMID: 17981659 Review.
-
U12-dependent intron splicing in plants.Curr Top Microbiol Immunol. 2008;326:61-82. doi: 10.1007/978-3-540-76776-3_4. Curr Top Microbiol Immunol. 2008. PMID: 18630747 Review.
Cited by
-
Splicing regulation in brain and testis: common themes for highly specialized organs.Cell Cycle. 2021 Mar-Mar;20(5-6):480-489. doi: 10.1080/15384101.2021.1889187. Epub 2021 Feb 26. Cell Cycle. 2021. PMID: 33632061 Free PMC article. Review.
-
Zrsr2 and functional U12-dependent spliceosome are necessary for follicular development.iScience. 2022 Feb 2;25(2):103860. doi: 10.1016/j.isci.2022.103860. eCollection 2022 Feb 18. iScience. 2022. PMID: 35198906 Free PMC article.
-
ZRSR1 co-operates with ZRSR2 in regulating splicing of U12-type introns in murine hematopoietic cells.Haematologica. 2022 Mar 1;107(3):680-689. doi: 10.3324/haematol.2020.260562. Haematologica. 2022. PMID: 33691379 Free PMC article.
-
Time-restricted feeding modulates the DNA methylation landscape, attenuates hallmark neuropathology and cognitive impairment in a mouse model of vascular dementia.Theranostics. 2022 Mar 21;12(7):3007-3023. doi: 10.7150/thno.71815. eCollection 2022. Theranostics. 2022. PMID: 35547760 Free PMC article.
-
Sequencing of the Pituitary Transcriptome after GnRH Treatment Uncovers the Involvement of lncRNA-m23b/miR-23b-3p/CAMK2D in FSH Synthesis and Secretion.Genes (Basel). 2023 Mar 31;14(4):846. doi: 10.3390/genes14040846. Genes (Basel). 2023. PMID: 37107604 Free PMC article.
References
-
- Horiuchi K., Perez-Cerezales S., Papasaikas P., Ramos-Ibeas P., Lopez-Cardona A.P., Laguna-Barraza R., Fonseca Balvis N., Pericuesta E., Fernandez-Gonzalez R., Planells B., et al. Impaired Spermatogenesis, Muscle, and Erythrocyte Function in U12 Intron Splicing-Defective Zrsr1 Mutant Mice. Cell Rep. 2018;23:143–155. doi: 10.1016/j.celrep.2018.03.028. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

