Ginsenoside Rb1 regulates prefrontal cortical GABAergic transmission in MPTP-treated mice
- PMID: 31314744
- PMCID: PMC6682523
- DOI: 10.18632/aging.102095
Ginsenoside Rb1 regulates prefrontal cortical GABAergic transmission in MPTP-treated mice
Abstract
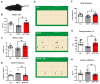
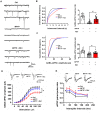
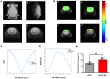
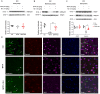
Parkinson's disease (PD) is a common neurodegenerative disease, featured by motor deficits and non-motor symptoms such as cognitive impairment, and malfunction of gamma-aminobutyric acid (GABA) mediated inhibitory transmission plays an important role in PD pathogenesis. The ginsenoside Rb1 molecule, a major constituent of the extract from the Ginseng root, has been demonstrated to ameliorate motor deficits and prevent dopaminergic neuron death in PD. However, whether Rb1 can regulate GABAergic transmission in PD-associated deficits and its underlying mechanisms are still unclear. In this study, we explored the effects of Rb1 on the GABAergic synaptic transmission in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD. We demonstrated that Rb1 can bind with GABAARα1 and increase its expression in the SH-SY5Y cells and in the prefrontal cortex (PFC) of MPTP model in vitro and in vivo. Furthermore, Rb1 can promote prefrontal cortical GABA level and GABAergic transmission in MPTP-treated mice. We also revealed that Rb1 may suppress presynaptic GABABR1 to enhance GABA release and GABAA receptor-mediated inhibitory transmission. In addition, Rb1 attenuated MPTP-induced dysfunctional gait dynamic and cognitive impairment, and this neuroprotective mechanism possibly involved regulating prefrontal cortical GABAergic transmission. Thus, Rb1 may serve as a potential drug candidate for the treatment of PD.
Keywords: GABA receptors; GABAergic transmission; Ginsenoside Rb1; Parkinson’s disease; cognitive impairment.
Conflict of interest statement
Figures



Similar articles
-
Ginsenoside Rb1 prevents MPTP-induced changes in hippocampal memory via regulation of the α-synuclein/PSD-95 pathway.Aging (Albany NY). 2019 Apr 4;11(7):1934-1964. doi: 10.18632/aging.101884. Aging (Albany NY). 2019. PMID: 30958793 Free PMC article.
-
Ginsenoside Rb1 confers neuroprotection via promotion of glutamate transporters in a mouse model of Parkinson's disease.Neuropharmacology. 2018 Mar 15;131:223-237. doi: 10.1016/j.neuropharm.2017.12.012. Epub 2017 Dec 12. Neuropharmacology. 2018. PMID: 29241654
-
Monoaminergic and aminoacidergic receptors are involved in the antidepressant-like effect of ginsenoside Rb1 in mouse hippocampus (CA3) and prefrontal cortex.Brain Res. 2018 Nov 15;1699:44-53. doi: 10.1016/j.brainres.2018.05.035. Epub 2018 May 23. Brain Res. 2018. PMID: 29802841
-
Prefrontal cortical gamma-aminobutyric acid transmission and cognitive function: drawing links to schizophrenia from preclinical research.Biol Psychiatry. 2015 Jun 1;77(11):929-39. doi: 10.1016/j.biopsych.2014.09.007. Epub 2014 Nov 18. Biol Psychiatry. 2015. PMID: 25442792 Review.
-
The intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a new rodent model to test palliative and neuroprotective agents for Parkinson's disease.Curr Pharm Des. 2011;17(5):489-507. doi: 10.2174/138161211795164095. Curr Pharm Des. 2011. PMID: 21375482 Review.
Cited by
-
Modified Huang-Lian-Jie-Du Decoction Ameliorates Aβ Synaptotoxicity in a Murine Model of Alzheimer's Disease.Oxid Med Cell Longev. 2019 Nov 3;2019:8340192. doi: 10.1155/2019/8340192. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31781354 Free PMC article.
-
Combined non-targeted and targeted metabolomics reveals the mechanism of delaying aging of Ginseng fibrous root.Front Pharmacol. 2024 Jul 24;15:1368776. doi: 10.3389/fphar.2024.1368776. eCollection 2024. Front Pharmacol. 2024. PMID: 39114359 Free PMC article.
-
Effects of Ginsenosides on Pentylenetetrazol-Induced Convulsions during Estrus Cycle in Rat.Arch Razi Inst. 2023 Aug 31;78(4):1359-1364. doi: 10.32592/ARI.2023.78.4.1359. eCollection 2023 Aug. Arch Razi Inst. 2023. PMID: 38226383 Free PMC article.
-
CNTNAP4 deficiency in dopaminergic neurons initiates parkinsonian phenotypes.Theranostics. 2020 Feb 10;10(7):3000-3021. doi: 10.7150/thno.40798. eCollection 2020. Theranostics. 2020. PMID: 32194851 Free PMC article.
-
Glutamic Acid Transporters: Targets for Neuroprotective Therapies in Parkinson's Disease.Front Neurosci. 2021 Jun 16;15:678154. doi: 10.3389/fnins.2021.678154. eCollection 2021. Front Neurosci. 2021. PMID: 34220434 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

