Angiotensin II mediates the axonal trafficking of tyrosine hydroxylase and dopamine β-hydroxylase mRNAs and enhances norepinephrine synthesis in primary sympathetic neurons
- PMID: 31306490
- PMCID: PMC7164330
- DOI: 10.1111/jnc.14821
Angiotensin II mediates the axonal trafficking of tyrosine hydroxylase and dopamine β-hydroxylase mRNAs and enhances norepinephrine synthesis in primary sympathetic neurons
Abstract
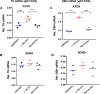
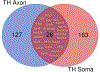
In the sympatho-adrenal system, angiotensin II (Ang II) acts as a key neuromodulatory component. At sympathetic nerve terminals, Ang II influences sympathetic transmission by enhancing norepinephrine (NE) synthesis, facilitating NE release and inhibiting NE uptake. Previously, it was demonstrated that tyrosine hydroxylase (TH) mRNA is trafficked to the distal axons of primary superior cervical ganglia (SCG) neurons, directed by a cis-acting regulatory element (i.e. zipcode) located in the 3'UTR of the transcript. Results of metabolic labeling studies established that the mRNA is locally translated. It was further shown that the axonal trafficking of the mRNA encoding the enzyme plays an important role in mediating dopamine (DA) and NE synthesis and may facilitate the maintenance of axonal catecholamine levels. In the present study, the hypothesis was tested that Ang II induces NE synthesis in rat primary SCG neurons via the modulation of the trafficking of the mRNAs encoding the catecholamine synthesizing enzymes TH and dopamine β-hydroxylase (DBH). Treatment of SCG neurons with the Ang II receptor type 1 (AT1R) agonist, L-162,313, increases the axonal levels of TH and DBH mRNA and protein and results in elevated NE levels. Conversely, treatment of rat SCG neurons with the AT1R antagonist, Eprosartan, abolished the L-162,313-mediated increase in axonal levels of TH and DBH mRNA and protein. In a first attempt to identify the proteins involved in the Ang II-mediated axonal transport of TH mRNA, we used a biotinylated 50-nucleotide TH RNA zipcode as bait in the affinity purification of TH zipcode-associated proteins. Mass spectrometric analysis of the TH zipcode ribonucleoprotein (RNP) complex immune-purified from SCG neurons led to the identification of 163 somal and 127 axonal proteins functionally involved in binding nucleic acids, the translational machinery or acting as subunits of cytoskeletal and motor proteins. Surprisingly, immune-purification of the TH axonal trafficking complex, results in the acquisition of DBH mRNA, suggesting that these mRNAs maybe transported to the axon together, possibly in the same RNP complex. Taken together, our results point to a novel mechanism by which Ang II participates in the regulation of axonal synthesis of NE by modulating the local trafficking and expression of TH and DBH, two key enzymes involved in the catecholamine biosynthetic pathway.
Keywords: axon; catecholamine; mRNA trafficking; superior cervical ganglia neurons; sympathetic nervous system; tyrosine hydroxylase.
© Published 2019. This article is a U.S. Government work and is in the public domain in the USA.
Conflict of interest statement
Conflicts of Interest
The authors declare no competing financial interests
Figures





Similar articles
-
Disruption of the Axonal Trafficking of Tyrosine Hydroxylase mRNA Impairs Catecholamine Biosynthesis in the Axons of Sympathetic Neurons.eNeuro. 2017 Jun 16;4(3):ENEURO.0385-16.2017. doi: 10.1523/ENEURO.0385-16.2017. eCollection 2017 May-Jun. eNeuro. 2017. PMID: 28630892 Free PMC article.
-
The local expression and trafficking of tyrosine hydroxylase mRNA in the axons of sympathetic neurons.RNA. 2016 Jun;22(6):883-95. doi: 10.1261/rna.053272.115. Epub 2016 Apr 19. RNA. 2016. PMID: 27095027 Free PMC article.
-
Adrenocorticotropic hormone elevates gene expression for catecholamine biosynthesis in rat superior cervical ganglia and locus coeruleus by an adrenal independent mechanism.Neuroscience. 2008 Jun 2;153(4):1380-9. doi: 10.1016/j.neuroscience.2008.02.059. Epub 2008 Mar 6. Neuroscience. 2008. PMID: 18440707 Free PMC article.
-
Genes for human catecholamine-synthesizing enzymes.Neurosci Res. 1991 Oct;12(2):315-45. doi: 10.1016/0168-0102(91)90001-f. Neurosci Res. 1991. PMID: 1684650 Review.
-
Molecular mechanisms behind mRNA localization in axons.Open Biol. 2020 Sep;10(9):200177. doi: 10.1098/rsob.200177. Epub 2020 Sep 23. Open Biol. 2020. PMID: 32961072 Free PMC article. Review.
Cited by
-
SARS-CoV-2 Dissemination Through Peripheral Nerves Explains Multiple Organ Injury.Front Cell Neurosci. 2020 Aug 5;14:229. doi: 10.3389/fncel.2020.00229. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32848621 Free PMC article.
-
Copper and the brain noradrenergic system.J Biol Inorg Chem. 2019 Dec;24(8):1179-1188. doi: 10.1007/s00775-019-01737-3. Epub 2019 Nov 5. J Biol Inorg Chem. 2019. PMID: 31691104 Free PMC article. Review.
-
The role of the brain renin-angiotensin system in Parkinson´s disease.Transl Neurodegener. 2024 Apr 15;13(1):22. doi: 10.1186/s40035-024-00410-3. Transl Neurodegener. 2024. PMID: 38622720 Free PMC article. Review.
-
Dopamine release and dopamine-related gene expression in the amygdala are modulated by the gastrin-releasing peptide in opposite directions during stress-enhanced fear learning and extinction.Mol Psychiatry. 2024 Nov 23. doi: 10.1038/s41380-024-02843-8. Online ahead of print. Mol Psychiatry. 2024. PMID: 39580604
References
-
- Berberich MJ, Kowalak JA, Makusky AJ, Martin B, Vullhorst D, Buonanno A, Markey SP (2011) Development of an On-Bead Digestion Procedure for Immunoprecipitated Proteins. Preparation in Biological Mass Spectrometry 109–124.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

