The α7 nicotinic receptor silent agonist R-47 prevents and reverses paclitaxel-induced peripheral neuropathy in mice without tolerance or altering nicotine reward and withdrawal
- PMID: 31299179
- PMCID: PMC6708482
- DOI: 10.1016/j.expneurol.2019.113010
The α7 nicotinic receptor silent agonist R-47 prevents and reverses paclitaxel-induced peripheral neuropathy in mice without tolerance or altering nicotine reward and withdrawal
Abstract
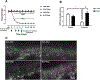
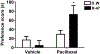
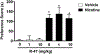
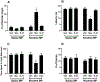
Various antitumor drugs, including paclitaxel, frequently cause chemotherapy-induced peripheral neuropathy (CIPN) that can be sustained even after therapy has been completed. The current work was designed to evaluate R-47, an α7 nAChR silent agonist, in our mouse model of CIPN. R-47 was administered to male C57BL/6J mice prior to and during paclitaxel treatment. Additionally, we tested if R-47 would alter nicotine's reward and withdrawal effects. The H460 and A549 non-small cell lung cancer (NSCLC) cell lines were exposed to R-47 for 24-72 h, and tumor-bearing NSG mice received R-47 prior to and during paclitaxel treatment. R-47 prevents and reverses paclitaxel-induced mechanical hypersensitivity in mice in an α7 nAChR-dependent manner. No tolerance develops following repeated administration of R-47, and the drug lacks intrinsic rewarding effects. Additionally, R-47 neither changes the rewarding effect of nicotine in the Conditioned Place Preference test nor enhances mecamylamine-precipitated withdrawal. Furthermore, R-47 prevents paclitaxel-mediated loss of intraepidermal nerve fibers and morphological alterations of microglia in the spinal cord. Moreover, R-47 does not increase NSCLC cell viability, colony formation, or proliferation, and does not interfere with paclitaxel-induced growth arrest, DNA fragmentation, or apoptosis. Most importantly, R-47 does not increase the growth of A549 tumors or interfere with the antitumor activity of paclitaxel in tumor-bearing mice. These studies suggest that R-47 could be a viable and efficacious approach for the prevention and treatment of CIPN that would not interfere with the antitumor activity of paclitaxel or promote lung tumor growth.
Keywords: Cancer; Mice; Neuropathy; Paclitaxel; Withdrawal; α7 nAChR.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest
The authors declare no conflicts of interest.
Figures


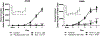
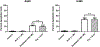
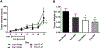
Similar articles
-
Nicotine Prevents and Reverses Paclitaxel-Induced Mechanical Allodynia in a Mouse Model of CIPN.J Pharmacol Exp Ther. 2018 Jan;364(1):110-119. doi: 10.1124/jpet.117.243972. Epub 2017 Oct 17. J Pharmacol Exp Ther. 2018. PMID: 29042416 Free PMC article.
-
Impact of modulation of the α7 nicotinic acetylcholine receptor on nicotine reward in the mouse conditioned place preference test.Psychopharmacology (Berl). 2019 Dec;236(12):3593-3599. doi: 10.1007/s00213-019-05331-y. Epub 2019 Jul 13. Psychopharmacology (Berl). 2019. PMID: 31302720 Free PMC article.
-
Pharmacological modulation of the α7 nicotinic acetylcholine receptor in a mouse model of mecamylamine-precipitated nicotine withdrawal.Psychopharmacology (Berl). 2018 Jul;235(7):1897-1905. doi: 10.1007/s00213-018-4879-7. Epub 2018 Mar 16. Psychopharmacology (Berl). 2018. PMID: 29549391 Free PMC article.
-
Involvement of α7 nAChR subtype in rat oxaliplatin-induced neuropathy: effects of selective activation.Neuropharmacology. 2014 Apr;79:37-48. doi: 10.1016/j.neuropharm.2013.10.034. Epub 2013 Nov 10. Neuropharmacology. 2014. PMID: 24225197
-
The Influence of Nicotine on Lung Tumor Growth, Cancer Chemotherapy, and Chemotherapy-Induced Peripheral Neuropathy.J Pharmacol Exp Ther. 2018 Aug;366(2):303-313. doi: 10.1124/jpet.118.249359. Epub 2018 Jun 4. J Pharmacol Exp Ther. 2018. PMID: 29866790 Free PMC article. Review.
Cited by
-
Centralizing the Knowledge and Interpretation of Pain in Chemotherapy-Induced Peripheral Neuropathy: A Paradigm Shift towards Brain-Centric Approaches.Brain Sci. 2024 Jun 28;14(7):659. doi: 10.3390/brainsci14070659. Brain Sci. 2024. PMID: 39061400 Free PMC article. Review.
-
Methods for in vivo studies in rodents of chemotherapy induced peripheral neuropathy.Exp Neurol. 2020 Mar;325:113154. doi: 10.1016/j.expneurol.2019.113154. Epub 2019 Dec 15. Exp Neurol. 2020. PMID: 31837318 Free PMC article. Review.
-
RgIA4 Accelerates Recovery from Paclitaxel-Induced Neuropathic Pain in Rats.Mar Drugs. 2019 Dec 21;18(1):12. doi: 10.3390/md18010012. Mar Drugs. 2019. PMID: 31877728 Free PMC article.
-
Exploring Cholinergic Compounds for Peripheral Neuropathic Pain Management: A Comprehensive Scoping Review of Rodent Model Studies.Pharmaceuticals (Basel). 2023 Sep 27;16(10):1363. doi: 10.3390/ph16101363. Pharmaceuticals (Basel). 2023. PMID: 37895835 Free PMC article.
-
Preclinical and Clinical Evidence of Therapeutic Agents for Paclitaxel-Induced Peripheral Neuropathy.Int J Mol Sci. 2021 Aug 13;22(16):8733. doi: 10.3390/ijms22168733. Int J Mol Sci. 2021. PMID: 34445439 Free PMC article. Review.
References
-
- Briggs CA, Grønlien JH, Curzon P, Timmermann DB, Ween H, Thorin-Hagene K, Kerr P, Anderson DJ, Malysz J, Dyhring T, Olsen GM, Peters D, Bunnelle WH, Gopalakrishnan M, 2009. Role of channel activation in cognitive enhancement mediated by alpha-7 nicotinic acetylcholine receptors. Br. J. Pharmacol 158, 1486–1494. doi:10.1111/j.1476-5381.2009.00426.x - DOI - PMC - PubMed
-
- Burgos E, Gómez-Nicola D, Pascual D, Martín I, Nieto-Sampedro M, Goicoechea C, 2012. Cannabinoid agonist WIN 55,212–2 prevents the development of paclitaxel-induced peripheral neuropathy in rats. Possible involvement of spinal glial cells. Eur. J. Pharmacol 682, 62–72. doi:10.1016/j.ejphar.2012.02.008 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

