Molecular foundations of primary spinal tumors-implications for surgical management
- PMID: 31297387
- PMCID: PMC6595199
- DOI: 10.21037/atm.2019.04.46
Molecular foundations of primary spinal tumors-implications for surgical management
Abstract
Primary spinal tumors are rare lesions that require careful clinical management due to their intimate relationship with critical neurovascular structures and the significant associated risk of morbidity. While the advent of molecular and genomic profiling is beginning to impact the management of the cranial counterparts, translation for spinal tumors has lagged behind. Maximal safe surgical resection remains the mainstay of patients with primary spinal tumors, with extent of resection and histology the only consistently identified independent predictors of survival. Adjuvant therapy has had limited impact. To develop targeted neoadjuvant and adjuvant therapies, improve prognostication, and enhance patient selection in spinal oncology, a thorough understanding of the current molecular and genomic landscape of spinal tumors is required. In this review, we detail the epidemiology, current standard-of-care, and molecular features of the most commonly encountered intramedullary spinal cord tumors (IMSCT), intradural extramedullary (IDEM) tumors, and primary spinal column malignancies (PSCM). We further discuss current efforts and future opportunities for integrating molecular advances in spinal oncology with clinical management.
Keywords: Intramedullary spinal cord tumor (IMSCT); chordoma; genomics; meningioma; schwannoma.
Conflict of interest statement
Conflicts of Interest: C Bettegowda is a consultant for Depuy-Synthes. The other authors have no conflicts of interest to declare.
Figures
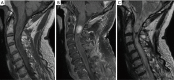
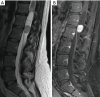
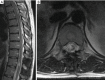
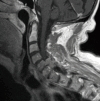
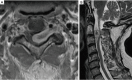
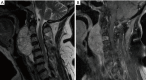
Similar articles
-
[Management of intramedullary spinal cord tumors: surgical considerations and results in 45 cases].Neurochirurgie. 2009 Jun;55(3):293-302. doi: 10.1016/j.neuchi.2008.02.060. Epub 2008 Jun 5. Neurochirurgie. 2009. PMID: 18538355 French.
-
Intramedullary Spinal Cord Tumors: Part II-Management Options and Outcomes.Global Spine J. 2016 Mar;6(2):176-85. doi: 10.1055/s-0035-1550086. Epub 2015 Jul 9. Global Spine J. 2016. PMID: 26933620 Free PMC article. Review.
-
Intradural spinal tumors in adults-update on management and outcome.Neurosurg Rev. 2019 Jun;42(2):371-388. doi: 10.1007/s10143-018-0957-x. Epub 2018 Feb 17. Neurosurg Rev. 2019. PMID: 29455369 Review.
-
Spinal cord and intradural-extraparenchymal spinal tumors: current best care practices and strategies.J Neurooncol. 2004 Aug-Sep;69(1-3):291-318. doi: 10.1023/b:neon.0000041889.71136.62. J Neurooncol. 2004. PMID: 15527097 Review.
-
Intramedullary Spinal Cord Tumors: Part I-Epidemiology, Pathophysiology, and Diagnosis.Global Spine J. 2015 Oct;5(5):425-35. doi: 10.1055/s-0035-1549029. Epub 2015 Mar 31. Global Spine J. 2015. PMID: 26430598 Free PMC article. Review.
Cited by
-
Spinal Meningiomas: A Comprehensive Review and Update on Advancements in Molecular Characterization, Diagnostics, Surgical Approach and Technology, and Alternative Therapies.Cancers (Basel). 2024 Apr 7;16(7):1426. doi: 10.3390/cancers16071426. Cancers (Basel). 2024. PMID: 38611105 Free PMC article. Review.
-
Surgical approaches to intramedullary spinal cord astrocytomas in the age of genomics.Front Oncol. 2022 Sep 6;12:982089. doi: 10.3389/fonc.2022.982089. eCollection 2022. Front Oncol. 2022. PMID: 36147920 Free PMC article. Review.
-
Predictive Analytics in Spine Oncology Research: First Steps, Limitations, and Future Directions.Neurospine. 2019 Dec;16(4):669-677. doi: 10.14245/ns.1938402.201. Epub 2019 Dec 31. Neurospine. 2019. PMID: 31905455 Free PMC article.
-
Neurological Outcome and Respiratory Insufficiency in Intramedullary Tumors of the Upper Cervical Spine.Medicina (Kaunas). 2023 Sep 30;59(10):1754. doi: 10.3390/medicina59101754. Medicina (Kaunas). 2023. PMID: 37893472 Free PMC article.
-
The disparity in pediatric spinal cord tumor clinical trials: A scoping review of registered clinical trials from 1989 to 2023.Neurooncol Pract. 2024 May 17;11(5):532-545. doi: 10.1093/nop/npae041. eCollection 2024 Oct. Neurooncol Pract. 2024. PMID: 39279782 Review.
References
Publication types
LinkOut - more resources
Full Text Sources
