A cysteinyl-tRNA synthetase variant confers resistance against selenite toxicity and decreases selenocysteine misincorporation
- PMID: 31296657
- PMCID: PMC6709638
- DOI: 10.1074/jbc.RA119.008219
A cysteinyl-tRNA synthetase variant confers resistance against selenite toxicity and decreases selenocysteine misincorporation
Abstract
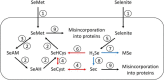
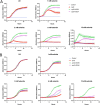
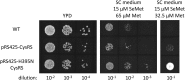
Selenocysteine (Sec) is the 21st genetically encoded amino acid in organisms across all domains of life. Although structurally similar to cysteine (Cys), the Sec selenol group has unique properties that are attractive for protein engineering and biotechnology applications. Production of designer proteins with Sec (selenoproteins) at desired positions is now possible with engineered translation systems in Escherichia coli However, obtaining pure selenoproteins at high yields is limited by the accumulation of free Sec in cells, causing undesired incorporation of Sec at Cys codons due to the inability of cysteinyl-tRNA synthetase (CysRS) to discriminate against Sec. Sec misincorporation is toxic to cells and causes protein aggregation in yeast. To overcome this limitation, here we investigated a CysRS from the selenium accumulator plant Astragalus bisulcatus that is reported to reject Sec in vitro Sequence analysis revealed a rare His → Asn variation adjacent to the CysRS catalytic pocket. Introducing this variation into E. coli and Saccharomyces cerevisiae CysRS increased resistance to the toxic effects of selenite and selenomethionine (SeMet), respectively. Although the CysRS variant could still use Sec as a substrate in vitro, we observed a reduction in the frequency of Sec misincorporation at Cys codons in vivo We surmise that the His → Asn variation can be introduced into any CysRS to provide a fitness advantage for strains burdened by Sec misincorporation and selenium toxicity. Our results also support the notion that the CysRS variant provides higher specificity for Cys as a mechanism for plants to grow in selenium-rich soils.
Keywords: Astragalus bisulcatus; Escherichia coli (E. coli); aminoacyl tRNA synthetase; cysteinyl-tRNA synthetase; protein engineering; selenite toxicity; selenium; selenocysteine; transfer RNA (tRNA); translation.
© 2019 Hoffman et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
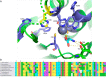

Similar articles
-
Recoding of the selenocysteine UGA codon by cysteine in the presence of a non-canonical tRNACys and elongation factor SelB.RNA Biol. 2018;15(4-5):471-479. doi: 10.1080/15476286.2018.1474074. Epub 2018 Jun 18. RNA Biol. 2018. PMID: 29879865 Free PMC article.
-
A dual-specificity aminoacyl-tRNA synthetase in the deep-rooted eukaryote Giardia lamblia.Proc Natl Acad Sci U S A. 2000 Nov 21;97(24):12997-3002. doi: 10.1073/pnas.230444397. Proc Natl Acad Sci U S A. 2000. PMID: 11078517 Free PMC article.
-
Cysteinyl-tRNA(Cys) formation in Methanocaldococcus jannaschii: the mechanism is still unknown.J Bacteriol. 2004 Jan;186(1):8-14. doi: 10.1128/JB.186.1.8-14.2004. J Bacteriol. 2004. PMID: 14679218 Free PMC article.
-
Challenges of site-specific selenocysteine incorporation into proteins by Escherichia coli.RNA Biol. 2018;15(4-5):461-470. doi: 10.1080/15476286.2018.1440876. Epub 2018 Mar 12. RNA Biol. 2018. PMID: 29447106 Free PMC article. Review.
-
Selenoproteins-What unique properties can arise with selenocysteine in place of cysteine?Exp Cell Res. 2010 May 1;316(8):1296-303. doi: 10.1016/j.yexcr.2010.02.032. Epub 2010 Mar 3. Exp Cell Res. 2010. PMID: 20206159 Review.
Cited by
-
Chemoproteomic interrogation of selenocysteine by low-pH isoTOP-ABPP.Methods Enzymol. 2022;662:187-225. doi: 10.1016/bs.mie.2021.10.003. Epub 2021 Nov 15. Methods Enzymol. 2022. PMID: 35101210 Free PMC article.
-
Scientific opinion on the tolerable upper intake level for selenium.EFSA J. 2023 Jan 20;21(1):e07704. doi: 10.2903/j.efsa.2023.7704. eCollection 2023 Jan. EFSA J. 2023. PMID: 36698500 Free PMC article.
-
Non-canonical Amino Acid Substrates of E. coli Aminoacyl-tRNA Synthetases.Chembiochem. 2022 Jan 5;23(1):e202100299. doi: 10.1002/cbic.202100299. Epub 2021 Sep 22. Chembiochem. 2022. PMID: 34416067 Free PMC article. Review.
-
Seleno-Amino Acids in Vegetables: A Review of Their Forms and Metabolism.Front Plant Sci. 2022 Feb 2;13:804368. doi: 10.3389/fpls.2022.804368. eCollection 2022. Front Plant Sci. 2022. PMID: 35185982 Free PMC article. Review.
-
HIF-1 Has a Central Role in Caenorhabditis elegans Organismal Response to Selenium.Front Genet. 2020 Feb 25;11:63. doi: 10.3389/fgene.2020.00063. eCollection 2020. Front Genet. 2020. PMID: 32161616 Free PMC article.
References
-
- Singh R., and Whitesides G. M. (1991) Selenols catalyze the interchange reactions of dithiols and disulfides in water. J. Org. Chem. 56, 6931–6933 10.1021/jo00024a041 - DOI
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

