The OsMPK15 Negatively Regulates Magnaporthe oryza and Xoo Disease Resistance via SA and JA Signaling Pathway in Rice
- PMID: 31293603
- PMCID: PMC6598650
- DOI: 10.3389/fpls.2019.00752
The OsMPK15 Negatively Regulates Magnaporthe oryza and Xoo Disease Resistance via SA and JA Signaling Pathway in Rice
Abstract
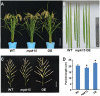
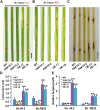
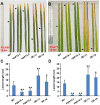
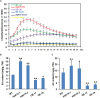
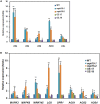
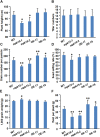
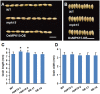
Mitogen-activated protein kinase (MAPK) cascades play central roles in response to biotic and abiotic stresses. However, the mechanisms by which various MAPK members regulate the plant immune response in rice remain elusive. In this article, to characterize the mechanisms, the knock-out and overexpression mutants of OsMPK15 were constructed and the disease resistance was investigated under the various fungal and bacterial inoculations. The knock-out mutant of OsMPK15 resulted in the constitutive expression of pathogenesis-related (PR) genes, increased accumulation of reactive oxygen species (ROS) triggered by the pathogen-associated molecular pattern (PAMP) elicitor chitin, and significantly enhanced the disease resistance to different races of Magnaporthe oryzae and Xanthomonas oryzae pv. oryzae (Xoo), which cause the rice blast and bacterial blight diseases, respectively. On contrary, the expression of PR genes and ROS were down-regulated in the OsMPK15-overexpressing (OsMPK15-OE) lines. Meanwhile, phytohormones such as salicylic acid (SA) and jasmonic acid (JA) were accumulated in the mpk15 mutant lines but decreased in the OsMPK15-OE lines. The expression of SA- and JA-pathway associated genes were significantly upregulated in the mpk15 mutant, whereas it was down regulated in the OsMPK15-OE lines. We conclude that OsMPK15 may negatively regulate the disease resistance through modulating SA- and JA-mediated signaling pathway.
Keywords: M. oryzae; OsMPK15; PRs; ROS; SA/JA; Xoo; rice.
Figures

Similar articles
-
Overexpression of MoSM1, encoding for an immunity-inducing protein from Magnaporthe oryzae, in rice confers broad-spectrum resistance against fungal and bacterial diseases.Sci Rep. 2017 Jan 20;7:41037. doi: 10.1038/srep41037. Sci Rep. 2017. PMID: 28106116 Free PMC article.
-
Jasmonic Acid, Not Salicyclic Acid Restricts Endophytic Root Colonization of Rice.Front Plant Sci. 2020 Jan 29;10:1758. doi: 10.3389/fpls.2019.01758. eCollection 2019. Front Plant Sci. 2020. PMID: 32063914 Free PMC article.
-
The group I GH3 family genes encoding JA-Ile synthetase act as positive regulator in the resistance of rice to Xanthomonas oryzae pv. oryzae.Biochem Biophys Res Commun. 2019 Jan 22;508(4):1062-1066. doi: 10.1016/j.bbrc.2018.12.057. Epub 2018 Dec 13. Biochem Biophys Res Commun. 2019. PMID: 30553449
-
Development of disease-resistant rice using regulatory components of induced disease resistance.Front Plant Sci. 2014 Nov 13;5:630. doi: 10.3389/fpls.2014.00630. eCollection 2014. Front Plant Sci. 2014. PMID: 25431577 Free PMC article. Review.
-
Mechanism of Rice Resistance to Bacterial Leaf Blight via Phytohormones.Plants (Basel). 2024 Sep 10;13(18):2541. doi: 10.3390/plants13182541. Plants (Basel). 2024. PMID: 39339516 Free PMC article. Review.
Cited by
-
CRISPR/Cas9-mediated VvPR4b editing decreases downy mildew resistance in grapevine (Vitis vinifera L.).Hortic Res. 2020 Sep 1;7:149. doi: 10.1038/s41438-020-00371-4. eCollection 2020. Hortic Res. 2020. PMID: 32922821 Free PMC article.
-
OsAPX1 Positively Contributes to Rice Blast Resistance.Front Plant Sci. 2022 Mar 21;13:843271. doi: 10.3389/fpls.2022.843271. eCollection 2022. Front Plant Sci. 2022. PMID: 35386681 Free PMC article.
-
Comparison of Transcriptome between Tolerant and Susceptible Rice Cultivar Reveals Positive and Negative Regulators of Response to Rhizoctonia solani in Rice.Int J Mol Sci. 2023 Sep 20;24(18):14310. doi: 10.3390/ijms241814310. Int J Mol Sci. 2023. PMID: 37762614 Free PMC article.
-
A Major Quantitative Trait Loci Cluster Controlling Three Components of Yield and Plant Height Identified on Chromosome 4B of Common Wheat.Front Plant Sci. 2022 Jan 11;12:799520. doi: 10.3389/fpls.2021.799520. eCollection 2021. Front Plant Sci. 2022. PMID: 35087558 Free PMC article.
-
Engineering plant immune circuit: walking to the bright future with a novel toolbox.Plant Biotechnol J. 2023 Jan;21(1):17-45. doi: 10.1111/pbi.13916. Epub 2022 Sep 27. Plant Biotechnol J. 2023. PMID: 36036862 Free PMC article. Review.
References
-
- Asai T., Tena G., Plotnikova J., Willmann M. R., Chiu W. L., Gomez-Gomez L., et al. (2002). MAP kinase signaling cascade in Arabidopsis innate immunity. Nature 415 977–983. - PubMed
-
- Brown J. K. (2002). Yield penalties of disease resistance in crops. Curr. Opin. Plant Biol. 5 339–344. - PubMed
-
- Century K. S., Shapiro A. D., Repetti P. P., Dahlbeck D., Holub E., Staskawicz B. J. (1997). NDR1, a pathogen-induced component required for Arabidopsis disease resistance. Science 278 1963–1965. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

