A Modified mRNA Vaccine Targeting Immunodominant NS Epitopes Protects Against Dengue Virus Infection in HLA Class I Transgenic Mice
- PMID: 31293584
- PMCID: PMC6598640
- DOI: 10.3389/fimmu.2019.01424
A Modified mRNA Vaccine Targeting Immunodominant NS Epitopes Protects Against Dengue Virus Infection in HLA Class I Transgenic Mice
Abstract
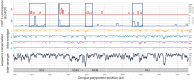
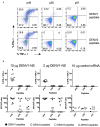
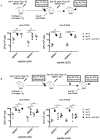
Dengue virus (DENV) induces strong T and B cell responses upon infection. Hence, it is difficult to determine the contribution of cell-mediated immunity alone in the long lasting protection against DENV infection and disease. Numerous CD4+ and CD8+ T cell epitopes have been identified, mainly in the non-structural proteins of DENV. Taking into account the immunogenicity and peptide sequence conservation among the different DENV serotypes, a minimal DENV antigen, called DENV1-NS, has been designed. This antigen is enriched in conserved and highly antigenic epitopes located in the NS3, NS4B, and NS5 regions of DENV1. To evaluate the ability of the DENV1-NS poly-epitope to express the antigenic peptides in the context of different HLA class I molecules, we established its in vivo immunogenicity by measuring, after DNA immunization and electroporation, the activation of DENV-specific CD8 T cells in transgenic mice expressing the human HLA-A*0201, -A*2402, -B*0702, and -B*3502 class I alleles. We then engineered a lipid nanoparticle (LNP) encapsulated modified mRNA vaccine encoding DENV1-NS and tested immunogenicity and protection in these human HLA class I transgenic mice, after transient blockade of the interferon (IFN) type I receptor. Significant protection was observed, after two injections of the mRNA vaccine. Collectively, these data strongly support the development of T cell-based vaccines targeting immunodominant T cell epitopes that generate potent virus-specific T cell responses conferring immunity against DENV infection.
Keywords: DNA vaccine; NS epitopes; T cells; chimeric vaccine; dengue virus (DENV); human HLA transgenic mice; vaccine.
Figures


Similar articles
-
A Dengue Virus Serotype 1 mRNA-LNP Vaccine Elicits Protective Immune Responses.J Virol. 2021 May 24;95(12):e02482-20. doi: 10.1128/JVI.02482-20. Print 2021 May 24. J Virol. 2021. PMID: 33762420 Free PMC article.
-
Identification and selection of immunodominant B and T cell epitopes for dengue multi-epitope-based vaccine.Med Microbiol Immunol. 2021 Feb;210(1):1-11. doi: 10.1007/s00430-021-00700-x. Epub 2021 Jan 30. Med Microbiol Immunol. 2021. PMID: 33515283 Review.
-
The human CD8+ T cell responses induced by a live attenuated tetravalent dengue vaccine are directed against highly conserved epitopes.J Virol. 2015 Jan;89(1):120-8. doi: 10.1128/JVI.02129-14. Epub 2014 Oct 15. J Virol. 2015. PMID: 25320311 Free PMC article.
-
Immunodominance changes as a function of the infecting dengue virus serotype and primary versus secondary infection.J Virol. 2014 Oct;88(19):11383-94. doi: 10.1128/JVI.01108-14. Epub 2014 Jul 23. J Virol. 2014. PMID: 25056881 Free PMC article.
-
Cross-Reactive T Cell Immunity to Dengue and Zika Viruses: New Insights Into Vaccine Development.Front Immunol. 2019 Jun 11;10:1316. doi: 10.3389/fimmu.2019.01316. eCollection 2019. Front Immunol. 2019. PMID: 31244855 Free PMC article. Review.
Cited by
-
Exposure of Platelets to Dengue Virus and Envelope Protein Domain III Induces Nlrp3 Inflammasome-Dependent Platelet Cell Death and Thrombocytopenia in Mice.Front Immunol. 2021 Apr 29;12:616394. doi: 10.3389/fimmu.2021.616394. eCollection 2021. Front Immunol. 2021. PMID: 33995345 Free PMC article.
-
Safety, Immunogenicity, and Mechanism of a Rotavirus mRNA-LNP Vaccine in Mice.Viruses. 2024 Jan 31;16(2):211. doi: 10.3390/v16020211. Viruses. 2024. PMID: 38399987 Free PMC article.
-
A Dengue Virus Serotype 1 mRNA-LNP Vaccine Elicits Protective Immune Responses.J Virol. 2021 May 24;95(12):e02482-20. doi: 10.1128/JVI.02482-20. Print 2021 May 24. J Virol. 2021. PMID: 33762420 Free PMC article.
-
Strategies for controlling the innate immune activity of conventional and self-amplifying mRNA therapeutics: Getting the message across.Adv Drug Deliv Rev. 2021 Sep;176:113900. doi: 10.1016/j.addr.2021.113900. Epub 2021 Jul 26. Adv Drug Deliv Rev. 2021. PMID: 34324884 Free PMC article. Review.
-
Messenger RNA-based vaccines: Past, present, and future directions in the context of the COVID-19 pandemic.Adv Drug Deliv Rev. 2021 Dec;179:114000. doi: 10.1016/j.addr.2021.114000. Epub 2021 Oct 9. Adv Drug Deliv Rev. 2021. PMID: 34637846 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

