Distinct Spatio-Temporal Dynamics of Tumor-Associated Neutrophils in Small Tumor Lesions
- PMID: 31293583
- PMCID: PMC6603174
- DOI: 10.3389/fimmu.2019.01419
Distinct Spatio-Temporal Dynamics of Tumor-Associated Neutrophils in Small Tumor Lesions
Abstract
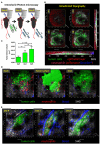
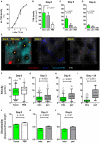
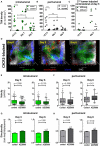
Across a majority of cancer types tumor-associated neutrophils (TAN) are linked with poor prognosis. However, the underlying mechanisms, especially the intratumoral behavior of TAN, are largely unknown. Using intravital multiphoton imaging on a mouse model with neutrophil-specific fluorescence, we measured the migration of TAN in distinct compartments of solid tumor cell lesions in vivo. By longitudinally quantifying the infiltration and persistence of TAN into growing tumors in the same animals, we observed cells that either populated the peripheral stromal zone of the tumor (peritumoral TAN) or infiltrated into the tumor core (intratumoral TAN). Intratumoral TAN showed prolonged tumor-associated persistence and reduced motility compared to peritumoral TAN, whose velocity increased with tumor progression. Selective pharmacological blockade of CXCR2 receptors using AZD5069 profoundly inhibited recruitment of TAN into peritumoral regions, while intratumoral infiltration was only transiently attenuated and rebounded at later time points. Our findings unravel distinct spatial dynamics of TAN that are partially and differentially regulated via the CXCR2 signaling pathway.
Keywords: AZD5069; CXCR2; intravital imaging; multiphoton microscopy; neutrophil granulocytes; tumor immunology; tumor microenvironment; tumor-associated neutrophils.
Figures
Similar articles
-
Effects of the CXCR2 antagonist AZD5069 on lung neutrophil recruitment in asthma.Pulm Pharmacol Ther. 2017 Aug;45:121-123. doi: 10.1016/j.pupt.2017.05.012. Epub 2017 May 23. Pulm Pharmacol Ther. 2017. PMID: 28549850 No abstract available.
-
Pharmacological characterization of AZD5069, a slowly reversible CXC chemokine receptor 2 antagonist.J Pharmacol Exp Ther. 2015 May;353(2):340-50. doi: 10.1124/jpet.114.221358. Epub 2015 Mar 3. J Pharmacol Exp Ther. 2015. PMID: 25736418
-
The effect of a selective CXCR2 antagonist (AZD5069) on human blood neutrophil count and innate immune functions.Br J Clin Pharmacol. 2015 Dec;80(6):1324-36. doi: 10.1111/bcp.12724. Epub 2015 Oct 2. Br J Clin Pharmacol. 2015. PMID: 26182832 Free PMC article. Clinical Trial.
-
Tumor-associated neutrophils: friend or foe?Carcinogenesis. 2012 May;33(5):949-55. doi: 10.1093/carcin/bgs123. Epub 2012 Mar 16. Carcinogenesis. 2012. PMID: 22425643 Review.
-
Human neutrophils: Their role in cancer and relation to myeloid-derived suppressor cells.Semin Immunol. 2016 Apr;28(2):187-96. doi: 10.1016/j.smim.2016.03.018. Epub 2016 Apr 7. Semin Immunol. 2016. PMID: 27067179 Review.
Cited by
-
Neutrophil dynamics in the tumor microenvironment.J Clin Invest. 2021 Mar 15;131(6):e143759. doi: 10.1172/JCI143759. J Clin Invest. 2021. PMID: 33720040 Free PMC article. Review.
-
Breast Cancer Cell-Neutrophil Interactions Enhance Neutrophil Survival and Pro-Tumorigenic Activities.Cancers (Basel). 2020 Oct 8;12(10):2884. doi: 10.3390/cancers12102884. Cancers (Basel). 2020. PMID: 33049964 Free PMC article.
-
Diverse Neutrophil Functions in Cancer and Promising Neutrophil-Based Cancer Therapies.Int J Mol Sci. 2022 Dec 13;23(24):15827. doi: 10.3390/ijms232415827. Int J Mol Sci. 2022. PMID: 36555469 Free PMC article. Review.
-
ICAM-1-suPAR-CD11b Axis Is a Novel Therapeutic Target for Metastatic Triple-Negative Breast Cancer.Cancers (Basel). 2023 May 12;15(10):2734. doi: 10.3390/cancers15102734. Cancers (Basel). 2023. PMID: 37345070 Free PMC article.
-
Tumor-Derived Factors Differentially Affect the Recruitment and Plasticity of Neutrophils.Cancers (Basel). 2021 Oct 11;13(20):5082. doi: 10.3390/cancers13205082. Cancers (Basel). 2021. PMID: 34680231 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

