Differential In Vitro Infection of Neural Cells by Astroviruses
- PMID: 31289185
- PMCID: PMC6747723
- DOI: 10.1128/mBio.01455-19
Differential In Vitro Infection of Neural Cells by Astroviruses
Abstract
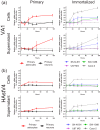
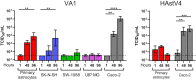
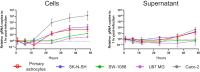
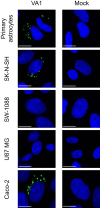
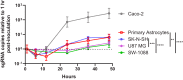
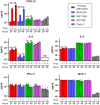
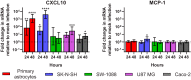
Recent advances in unbiased pathogen discovery have implicated astroviruses as pathogens of the central nervous system (CNS) of mammals, including humans. However, the capacity of astroviruses to be cultured in CNS-derived cells in vitro has not been reported to date. Both astrovirus VA1/HMO-C (VA1; mamastrovirus 9) and classic human astrovirus 4 (HAstV4; mamastrovirus 1) have been previously detected from cases of human encephalitis. We tested the ability of primary human neurons, primary human astrocytes, and other immortalized human nervous system cell lines (SK-N-SH, U87 MG, and SW-1088) to support infection and replication of these two astrovirus genotypes. Primary astrocytes and SK-N-SH cells supported the full viral life cycle of VA1 with a >100-fold increase in viral RNA levels during a multistep growth curve, detection of viral capsid, and a >100-fold increase in viral titer. Primary astrocytes were permissive with respect to HAstV4 infection and replication but did not yield infectious virus, suggesting abortive infection. Similarly, abortive infection of VA1 was observed in SW-1088 and U87 MG cells. Elevated expression of the chemokine CXCL10 was detected in VA1-infected primary astrocytes and SK-N-SH cells, suggesting that VA1 infection can induce a proinflammatory host response. These findings establish an in vitro cell culture model that is essential for investigation of the basic biology of astroviruses and their neuropathogenic potential.IMPORTANCE Encephalitis remains a diagnostic conundrum in humans as over 50% of cases are managed without the identification of an etiology. Astroviruses have been detected from the central nervous system of mammals in association with disease, suggesting that this family of RNA viruses could be responsible for cases of some neurological diseases that are currently without an ascribed etiology. However, there are significant barriers to understanding astrovirus infection as the capacity of these viruses to replicate in nervous system cells in vitro has not been determined. We describe primary and immortalized cultured cells of the nervous system that support infection by astroviruses. These results further corroborate the role of astroviruses in causing neurological diseases and will serve as an essential model to interrogate the neuropathogenesis of astrovirus infection.
Keywords: astrovirus; astrovirus VA1; cell culture; encephalitis; virology.
Copyright © 2019 Janowski et al.
Figures
Similar articles
-
Propagation of Astrovirus VA1, a Neurotropic Human Astrovirus, in Cell Culture.J Virol. 2017 Sep 12;91(19):e00740-17. doi: 10.1128/JVI.00740-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28701405 Free PMC article.
-
Antiviral activity of ribavirin and favipiravir against human astroviruses.J Clin Virol. 2020 Feb;123:104247. doi: 10.1016/j.jcv.2019.104247. Epub 2019 Dec 17. J Clin Virol. 2020. PMID: 31864069 Free PMC article.
-
Human Astrovirus MLB Replication In Vitro: Persistence in Extraintestinal Cell Lines.J Virol. 2019 Jun 14;93(13):e00557-19. doi: 10.1128/JVI.00557-19. Print 2019 Jul 1. J Virol. 2019. PMID: 31019055 Free PMC article.
-
Human Astrovirus VA1 Encephalitis in Pediatric Patients With Cancer: Report of 2 Cases and Review of the Literature.J Pediatric Infect Dis Soc. 2022 Sep 29;11(9):408-412. doi: 10.1093/jpids/piac045. J Pediatric Infect Dis Soc. 2022. PMID: 35849135 Review.
-
[Reseanh advance in human astrovirus].Bing Du Xue Bao. 2012 Jun;28(4):482-7. Bing Du Xue Bao. 2012. PMID: 22978177 Review. Chinese.
Cited by
-
Astrovirus replication in human intestinal enteroids reveals multi-cellular tropism and an intricate host innate immune landscape.PLoS Pathog. 2019 Oct 31;15(10):e1008057. doi: 10.1371/journal.ppat.1008057. eCollection 2019 Oct. PLoS Pathog. 2019. PMID: 31671153 Free PMC article.
-
Astrovirus-Associated Polioencephalomyelitis in an Alpaca.Viruses. 2020 Dec 30;13(1):50. doi: 10.3390/v13010050. Viruses. 2020. PMID: 33396858 Free PMC article.
-
Beyond the Gastrointestinal Tract: The Emerging and Diverse Tissue Tropisms of Astroviruses.Viruses. 2021 Apr 22;13(5):732. doi: 10.3390/v13050732. Viruses. 2021. PMID: 33922259 Free PMC article. Review.
-
Pathogenesis of Chicken Astrovirus Related Illnesses.Front Vet Sci. 2022 Jun 10;9:899901. doi: 10.3389/fvets.2022.899901. eCollection 2022. Front Vet Sci. 2022. PMID: 35754540 Free PMC article. Review.
-
Attenuation hotspots in neurotropic human astroviruses.PLoS Biol. 2023 Jul 17;21(7):e3001815. doi: 10.1371/journal.pbio.3001815. eCollection 2023 Jul. PLoS Biol. 2023. PMID: 37459343 Free PMC article.
References
-
- Ambrose HE, Granerod J, Clewley JP, Davies NW, Keir G, Cunningham R, Zuckerman M, Mutton KJ, Ward KN, Ijaz S, Crowcroft NS, Brown DW; UK Aetiology of Encephalitis Study Group. 2011. Diagnostic strategy used to establish etiologies of encephalitis in a prospective cohort of patients in England. J Clin Microbiol 49:3576–3583. doi:10.1128/JCM.00862-11. - DOI - PMC - PubMed
-
- Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA. 2012. The frequency of autoimmune N-methyl-d-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clin Infect Dis 54:899–904. doi:10.1093/cid/cir1038. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

