Expandable human cardiovascular progenitors from stem cells for regenerating mouse heart after myocardial infarction
- PMID: 31287499
- PMCID: PMC7252440
- DOI: 10.1093/cvr/cvz181
Expandable human cardiovascular progenitors from stem cells for regenerating mouse heart after myocardial infarction
Abstract
Aims: Cardiovascular diseases caused by loss of functional cardiomyocytes (CMs) are a major cause of mortality and morbidity worldwide due in part to the low regenerative capacity of the adult human heart. Human pluripotent stem cell (hPSC)-derived cardiovascular progenitor cells (CPCs) are a potential cell source for cardiac repair. The aim of this study was to examine the impact of extensive remuscularization and coincident revascularization on cardiac remodelling and function in a mouse model of myocardial infarction (MI) by transplanting doxycycline (DOX)-inducible (Tet-On-MYC) hPSC-derived CPCs in vivo and inducing proliferation and cardiovascular differentiation in a drug-regulated manner.
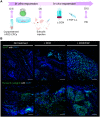
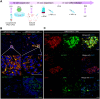
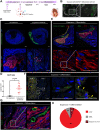
Methods and results: CPCs were injected firstly at a non-cardiac site in Matrigel suspension under the skin of immunocompromised mice to assess their commitment to the cardiovascular lineage and ability to self-renew or differentiate in vivo when instructed by systemically delivered factors including DOX and basic fibroblast growth factor (bFGF). CPCs in Matrigel were then injected intra-myocardially in mice subjected to MI to assess whether expandable CPCs could mediate cardiac repair. Transplanted CPCs expanded robustly both subcutis and in the myocardium using the same DOX/growth factor inducing regime. Upon withdrawal of these cell-renewal factors, CPCs differentiated with high efficiency at both sites into the major cardiac lineages including CMs, endothelial cells, and smooth muscle cells. After MI, engraftment of CPCs in the heart significantly reduced fibrosis in the infarcted area and prevented left ventricular remodelling, although cardiac function determined by magnetic resonance imaging was unaltered.
Conclusion: Replacement of large areas of muscle may be required to regenerate the heart of patients following MI. Our human/mouse model demonstrated that proliferating hPSC-CPCs could reduce infarct size and fibrosis resulting in formation of large grafts. Importantly, the results suggested that expanding transplanted cells in situ at the progenitor stage maybe be an effective alternative causing less tissue damage than injection of very large numbers of CMs.
Keywords: Human pluripotent stem cells; Expandable human cardiovascular progenitors; Mouse model; Myocardial infarction; Transplantation.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures
Similar articles
-
Irisin promotes cardiac progenitor cell-induced myocardial repair and functional improvement in infarcted heart.J Cell Physiol. 2019 Feb;234(2):1671-1681. doi: 10.1002/jcp.27037. Epub 2018 Sep 1. J Cell Physiol. 2019. PMID: 30171682 Free PMC article.
-
N-cadherin overexpression enhances the reparative potency of human-induced pluripotent stem cell-derived cardiac myocytes in infarcted mouse hearts.Cardiovasc Res. 2020 Mar 1;116(3):671-685. doi: 10.1093/cvr/cvz179. Cardiovasc Res. 2020. PMID: 31350544 Free PMC article.
-
Apoptosis-Resistant Cardiac Progenitor Cells Modified With Apurinic/Apyrimidinic Endonuclease/Redox Factor 1 Gene Overexpression Regulate Cardiac Repair After Myocardial Infarction.Stem Cells Transl Med. 2016 Aug;5(8):1067-78. doi: 10.5966/sctm.2015-0281. Epub 2016 Jun 22. Stem Cells Transl Med. 2016. PMID: 27334489 Free PMC article.
-
HiPS-Cardiac Trilineage Cell Generation and Transplantation: a Novel Therapy for Myocardial Infarction.J Cardiovasc Transl Res. 2020 Feb;13(1):110-119. doi: 10.1007/s12265-019-09891-4. Epub 2019 May 31. J Cardiovasc Transl Res. 2020. PMID: 31152358 Review.
-
Embryonic template-based generation and purification of pluripotent stem cell-derived cardiomyocytes for heart repair.J Cardiovasc Transl Res. 2012 Oct;5(5):566-80. doi: 10.1007/s12265-012-9391-6. Epub 2012 Jul 18. J Cardiovasc Transl Res. 2012. PMID: 22806916 Review.
Cited by
-
Functional enhancement of acute infracted heart by coinjection of autologous adipose-derived stem cells with matrigel.Turk J Biol. 2023 May 4;47(3):170-185. doi: 10.55730/1300-0152.2653. eCollection 2023. Turk J Biol. 2023. PMID: 37529419 Free PMC article.
-
Neuregulin-1, a potential therapeutic target for cardiac repair.Front Pharmacol. 2022 Aug 31;13:945206. doi: 10.3389/fphar.2022.945206. eCollection 2022. Front Pharmacol. 2022. PMID: 36120374 Free PMC article. Review.
-
Cell Therapy With Human ESC-Derived Cardiac Cells: Clinical Perspectives.Front Bioeng Biotechnol. 2020 Oct 26;8:601560. doi: 10.3389/fbioe.2020.601560. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33195177 Free PMC article. Review.
-
Cleistopholis patens root bark extract exerts cardioprotective effect against doxorubicin-induced myocardial toxicity in rats.Lab Anim Res. 2024 Nov 18;40(1):39. doi: 10.1186/s42826-024-00225-3. Lab Anim Res. 2024. PMID: 39551811 Free PMC article.
-
YTHDC1 Mitigates Apoptosis in Bone Marrow Mesenchymal Stem Cells by Inhibiting NfƙBiα and Augmenting Cardiac Function Following Myocardial Infarction.Cell Transplant. 2024 Jan-Dec;33:9636897241290910. doi: 10.1177/09636897241290910. Cell Transplant. 2024. PMID: 39466658 Free PMC article.
References
-
- Laflamme MA, Murry CE.. Regenerating the heart. Nat Biotechnol 2005;23:845.. - PubMed
-
- Kempf H, Andree B, Zweigerdt R.. Large-scale production of human pluripotent stem cell derived cardiomyocytes. Adv Drug Deliv Rev 2016;96:18–30. - PubMed
-
- Chong JJH, Yang X, Don CW, Minami E, Liu Y-W, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem H-P, Laflamme MA, Murry CE.. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014;510:273.. - PMC - PubMed
-
- Birket MJ, Ribeiro MC, Verkerk AO, Ward D, Leitoguinho AR, den Hartogh SC, Orlova VV, Devalla HD, Schwach V, Bellin M, Passier R, Mummery CL.. Expansion and patterning of cardiovascular progenitors derived from human pluripotent stem cells. Nat Biotechnol 2015;33:970.. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

