Strigolactones Play an Important Role in Shaping Exodermal Morphology via a KAI2-Dependent Pathway
- PMID: 31276958
- PMCID: PMC6611997
- DOI: 10.1016/j.isci.2019.06.024
Strigolactones Play an Important Role in Shaping Exodermal Morphology via a KAI2-Dependent Pathway
Abstract
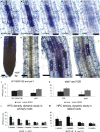
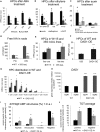
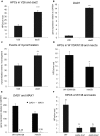
The majority of land plants have two suberized root barriers: the endodermis and the hypodermis (exodermis). Both barriers bear non-suberized passage cells that are thought to regulate water and nutrient exchange between the root and the soil. We learned a lot about endodermal passage cells, whereas our knowledge on hypodermal passage cells (HPCs) is still very scarce. Here we report on factors regulating the HPC number in Petunia roots. Strigolactones exhibit a positive effect, whereas supply of abscisic acid (ABA), ethylene, and auxin result in a strong reduction of the HPC number. Unexpectedly the strigolactone signaling mutant d14/dad2 showed significantly higher HPC numbers than the wild-type. In contrast, its mutant counterpart max2 of the heterodimeric receptor DAD2/MAX2 displayed a significant decrease in HPC number. A mutation in the Petunia karrikin sensor KAI2 exhibits drastically decreased HPC amounts, supporting the hypothesis that the dimeric KAI2/MAX2 receptor is central in determining the HPC number.
Keywords: Biological Sciences; Molecular Plant Pathology; Plant Biology; Plant Physiology.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interest.
Figures
Similar articles
-
Comparing and Contrasting the Multiple Roles of Butenolide Plant Growth Regulators: Strigolactones and Karrikins in Plant Development and Adaptation to Abiotic Stresses.Int J Mol Sci. 2019 Dec 12;20(24):6270. doi: 10.3390/ijms20246270. Int J Mol Sci. 2019. PMID: 31842355 Free PMC article. Review.
-
Evidence that KARRIKIN-INSENSITIVE2 (KAI2) Receptors may Perceive an Unknown Signal that is not Karrikin or Strigolactone.Front Plant Sci. 2016 Jan 8;6:1219. doi: 10.3389/fpls.2015.01219. eCollection 2015. Front Plant Sci. 2016. PMID: 26779242 Free PMC article.
-
Strigolactones, karrikins and beyond.Plant Cell Environ. 2017 Sep;40(9):1691-1703. doi: 10.1111/pce.12996. Epub 2017 Jul 5. Plant Cell Environ. 2017. PMID: 28558130 Review.
-
Evolution of strigolactone receptors by gradual neo-functionalization of KAI2 paralogues.BMC Biol. 2017 Jun 29;15(1):52. doi: 10.1186/s12915-017-0397-z. BMC Biol. 2017. PMID: 28662667 Free PMC article.
-
Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis.Development. 2012 Apr;139(7):1285-95. doi: 10.1242/dev.074567. Epub 2012 Feb 22. Development. 2012. PMID: 22357928
Cited by
-
Evolution of small molecule-mediated regulation of arbuscular mycorrhiza symbiosis.Philos Trans R Soc Lond B Biol Sci. 2024 Nov 18;379(1914):20230369. doi: 10.1098/rstb.2023.0369. Epub 2024 Sep 30. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 39343030 Free PMC article. Review.
-
A roadmap of plant membrane transporters in arbuscular mycorrhizal and legume-rhizobium symbioses.Plant Physiol. 2021 Dec 4;187(4):2071-2091. doi: 10.1093/plphys/kiab280. Plant Physiol. 2021. PMID: 34618047 Free PMC article. Review.
-
Conditioning plants for arbuscular mycorrhizal symbiosis through DWARF14-LIKE signalling.Curr Opin Plant Biol. 2021 Aug;62:102071. doi: 10.1016/j.pbi.2021.102071. Epub 2021 Jun 26. Curr Opin Plant Biol. 2021. PMID: 34186295 Free PMC article. Review.
-
The karrikin signaling regulator SMAX1 controls Lotus japonicus root and root hair development by suppressing ethylene biosynthesis.Proc Natl Acad Sci U S A. 2020 Sep 1;117(35):21757-21765. doi: 10.1073/pnas.2006111117. Epub 2020 Aug 17. Proc Natl Acad Sci U S A. 2020. PMID: 32817510 Free PMC article.
-
Comparing and Contrasting the Multiple Roles of Butenolide Plant Growth Regulators: Strigolactones and Karrikins in Plant Development and Adaptation to Abiotic Stresses.Int J Mol Sci. 2019 Dec 12;20(24):6270. doi: 10.3390/ijms20246270. Int J Mol Sci. 2019. PMID: 31842355 Free PMC article. Review.
References
-
- Al-Babili S., Bouwmeester H.J. Strigolactones, a novel carotenoid-derived plant hormone. Annu. Rev. Plant Biol. 2015;66:161–186. - PubMed
-
- Barberon M., Vermeer J.E., De Bellis D., Wang P., Naseer S., Andersen T.G., Humbel B.M., Nawrath C., Takano J., Salt D.E., Geldner N. Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell. 2016;164:447–459. - PubMed
LinkOut - more resources
Full Text Sources

