Immune-Mediated Control of a Dormant Neurotropic RNA Virus Infection
- PMID: 31270232
- PMCID: PMC6714802
- DOI: 10.1128/JVI.00241-19
Immune-Mediated Control of a Dormant Neurotropic RNA Virus Infection
Abstract
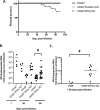
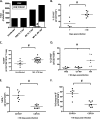
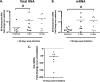
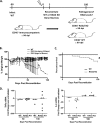
Genomic material from many neurotropic RNA viruses (e.g., measles virus [MV], West Nile virus [WNV], Sindbis virus [SV], rabies virus [RV], and influenza A virus [IAV]) remains detectable in the mouse brain parenchyma long after resolution of the acute infection. The presence of these RNAs in the absence of overt central nervous system (CNS) disease has led to the suggestion that they are viral remnants, with little or no potential to reactivate. Here we show that MV RNA remains detectable in permissive mouse neurons long after challenge with MV and, moreover, that immunosuppression can cause RNA and protein synthesis to rebound, triggering neuropathogenesis months after acute viral control. Robust recrudescence of viral transcription and protein synthesis occurs after experimental depletion of cells of the adaptive immune response and is associated with a loss of T resident memory (Trm) lymphocytes within the brain. The disease associated with loss of immune control is distinct from that seen during the acute infection: immune cell-depleted, long-term-infected mice display severe gait and motor problems, in contrast to the wasting and lethal disease that occur during acute infection of immunodeficient hosts. These results illuminate the potential consequences of noncytolytic, immune-mediated viral control in the CNS and demonstrate that what were once considered "resolved" RNA viral infections may, in fact, induce diseases later in life that are distinct from those caused by acute infection.IMPORTANCE Viral infections of neurons are often not cytopathic; thus, once-infected neurons survive, and viral RNAs can be detected long after apparent viral control. These RNAs are generally considered viral fossils, unlikely to contribute to central nervous system (CNS) disease. Using a mouse model of measles virus (MV) neuronal infection, we show that MV RNA is maintained in the CNS of infected mice long after acute control and in the absence of overt disease. Viral replication is suppressed by the adaptive immune response; when these immune cells are depleted, viral protein synthesis recurs, inducing a CNS disease that is distinct from that observed during acute infection. The studies presented here provide the basis for understanding how persistent RNA infections in the CNS are controlled by the host immune response, as well as the pathogenic consequences of noncytolytic viral control.
Keywords: RNA virus; T resident memory cells; central nervous system; measles virus; neuron; viral persistence.
Copyright © 2019 American Society for Microbiology.
Figures



Similar articles
-
Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent.J Virol. 2002 May;76(9):4497-506. doi: 10.1128/jvi.76.9.4497-4506.2002. J Virol. 2002. PMID: 11932415 Free PMC article.
-
Alpha-Synuclein Expression Restricts RNA Viral Infections in the Brain.J Virol. 2015 Dec 30;90(6):2767-82. doi: 10.1128/JVI.02949-15. J Virol. 2015. PMID: 26719256 Free PMC article.
-
A mouse model of persistent brain infection with recombinant Measles virus.J Gen Virol. 2006 Jul;87(Pt 7):2011-2019. doi: 10.1099/vir.0.81838-0. J Gen Virol. 2006. PMID: 16760404
-
Neuronal survival strategies in the face of RNA viral infection.J Infect Dis. 2002 Dec 1;186 Suppl 2(Suppl 2):S215-9. doi: 10.1086/344265. J Infect Dis. 2002. PMID: 12424700 Free PMC article. Review.
-
Making it to the synapse: measles virus spread in and among neurons.Curr Top Microbiol Immunol. 2009;330:3-30. doi: 10.1007/978-3-540-70617-5_1. Curr Top Microbiol Immunol. 2009. PMID: 19203102 Free PMC article. Review.
Cited by
-
Targeting Infectious Agents as a Therapeutic Strategy in Alzheimer's Disease.CNS Drugs. 2020 Jul;34(7):673-695. doi: 10.1007/s40263-020-00737-1. CNS Drugs. 2020. PMID: 32458360 Free PMC article.
-
The Influence of Virus Infection on Microglia and Accelerated Brain Aging.Cells. 2021 Jul 20;10(7):1836. doi: 10.3390/cells10071836. Cells. 2021. PMID: 34360004 Free PMC article. Review.
-
Why does viral RNA sometimes persist after recovery from acute infections?PLoS Biol. 2022 Jun 1;20(6):e3001687. doi: 10.1371/journal.pbio.3001687. eCollection 2022 Jun. PLoS Biol. 2022. PMID: 35648781 Free PMC article.
-
Multiple Receptors Involved in Invasion and Neuropathogenicity of Canine Distemper Virus: A Review.Viruses. 2022 Jul 12;14(7):1520. doi: 10.3390/v14071520. Viruses. 2022. PMID: 35891500 Free PMC article. Review.
-
Coronavirus persistence in human respiratory tract and cell culture: An overview.Braz J Infect Dis. 2021 Sep-Oct;25(5):101632. doi: 10.1016/j.bjid.2021.101632. Epub 2021 Oct 2. Braz J Infect Dis. 2021. PMID: 34627782 Free PMC article. Review.
References
-
- Li W, Lee M-H, Henderson L, Tyagi R, Bachani M, Steiner J, Campanac E, Hoffman DA, Geldern von G, Johnson K, Maric D, Morris HD, Lentz M, Pak K, Mammen A, Ostrow L, Rothstein J, Nath A. 2015. Human endogenous retrovirus-K contributes to motor neuron disease. Sci Transl Med 7:307ra153. doi:10.1126/scitranslmed.aac8201. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

