Fumonisin B1 Induces Oxidative Stress and Breaks Barrier Functions in Pig Iliac Endothelium Cells
- PMID: 31269688
- PMCID: PMC6669581
- DOI: 10.3390/toxins11070387
Fumonisin B1 Induces Oxidative Stress and Breaks Barrier Functions in Pig Iliac Endothelium Cells
Abstract
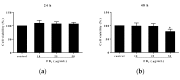
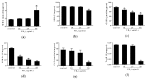
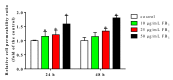
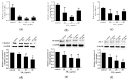
Fumonisins (Fums) are types of mycotoxin that widely contaminante feed material crops, and can trigger potential biological toxicities to humans and various animals. However, the toxicity of Fums on porcine blood vessels has not been fully explored. Fumonisin B1 (FB1) is the main component of Fums. Therefore, the aim of this study was to explore the effects of FB1 on the oxidative stress and tight junctions of the pig iliac endothelial cells (PIECs) in vitro. The results showed that FB1 reduced the viability of PIECs, increased the contents of lipid peroxidation product malondialdehyde (MDA), decreased the activities of antioxidant enzymes superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT) and thioredoxin reductase (TrxR), and decreased the level of glutathione (GSH). In addition, the barrier functions were destroyed, along with the down-regulations on Claudin 1, Occludin and ZO-1 and the increase of paracellular permeability. Thus, this research indicates that FB1 facilitates oxidative stress and breaks barrier functions to damage pig iliac endothelium cells.
Keywords: Fumonisin B1; oxidative stress; pig iliac endothelial cells; tight junction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Fumonisin B1 Inhibits Cell Proliferation and Decreases Barrier Function of Swine Umbilical Vein Endothelial Cells.Toxins (Basel). 2021 Dec 3;13(12):863. doi: 10.3390/toxins13120863. Toxins (Basel). 2021. PMID: 34941701 Free PMC article.
-
Fumonisin B1 damages the barrier functions of porcine intestinal epithelial cells in vitro.J Biochem Mol Toxicol. 2019 Nov;33(11):e22397. doi: 10.1002/jbt.22397. Epub 2019 Sep 26. J Biochem Mol Toxicol. 2019. PMID: 31557363
-
Interaction of aflatoxin B1 and fumonisin B1 in mice causes immunotoxicity and oxidative stress: Possible protective role using lactic acid bacteria.J Immunotoxicol. 2016;13(1):46-54. doi: 10.3109/1547691X.2014.997905. Epub 2015 Jan 14. J Immunotoxicol. 2016. PMID: 25585958
-
Fumonisin B1 induces oxidative stress in oesophageal (SNO) cancer cells.Toxicon. 2018 Jan;141:104-111. doi: 10.1016/j.toxicon.2017.12.041. Epub 2017 Dec 9. Toxicon. 2018. PMID: 29233736
-
Fumonisin distorts the cellular membrane lipid profile: A mechanistic insight.Toxicology. 2024 Aug;506:153860. doi: 10.1016/j.tox.2024.153860. Epub 2024 Jun 12. Toxicology. 2024. PMID: 38871209 Review.
Cited by
-
Toxic Mechanism and Biological Detoxification of Fumonisins.Toxins (Basel). 2022 Mar 1;14(3):182. doi: 10.3390/toxins14030182. Toxins (Basel). 2022. PMID: 35324679 Free PMC article. Review.
-
Role of ferroptosis in food-borne mycotoxin-induced toxicities.Apoptosis. 2024 Apr;29(3-4):267-276. doi: 10.1007/s10495-023-01907-4. Epub 2023 Nov 24. Apoptosis. 2024. PMID: 38001339 Review.
-
The Molecular Mechanisms of Ferroptosis and Its Role in Blood-Brain Barrier Dysfunction.Front Cell Neurosci. 2022 May 19;16:889765. doi: 10.3389/fncel.2022.889765. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35663422 Free PMC article. Review.
-
Evaluation of the Individual and Combined Toxicity of Fumonisin Mycotoxins in Human Gastric Epithelial Cells.Int J Mol Sci. 2020 Aug 18;21(16):5917. doi: 10.3390/ijms21165917. Int J Mol Sci. 2020. PMID: 32824643 Free PMC article.
-
Aptamer-Target-Gold Nanoparticle Conjugates for the Quantification of Fumonisin B1.Biosensors (Basel). 2021 Jan 8;11(1):18. doi: 10.3390/bios11010018. Biosensors (Basel). 2021. PMID: 33430067 Free PMC article.
References
-
- Ahangarkani F., Rouhi S., Gholamour Azizi I. A review on incidence and toxicity of fumonisins. Toxin Rev. 2014;33:95–100. doi: 10.3109/15569543.2013.871563. - DOI
-
- Upadhaya S.D., Park M.A., Ha J.K. Mycotoxins and Their Biotransformation in the Rumen: A Review. Asian Austral. J. Anim. Sci. 2010;23:1250–1260. doi: 10.5713/ajas.2010.r.06. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

