Targeting Cell Adhesion Molecules via Carbonate Apatite-Mediated Delivery of Specific siRNAs to Breast Cancer Cells In Vitro and In Vivo
- PMID: 31269666
- PMCID: PMC6680929
- DOI: 10.3390/pharmaceutics11070309
Targeting Cell Adhesion Molecules via Carbonate Apatite-Mediated Delivery of Specific siRNAs to Breast Cancer Cells In Vitro and In Vivo
Abstract
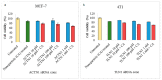
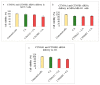
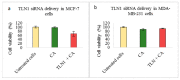
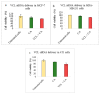
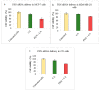
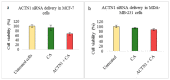
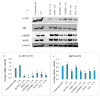
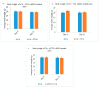
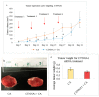
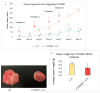
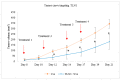
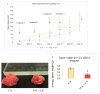
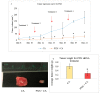
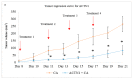
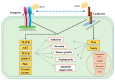
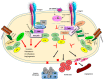
While several treatment strategies are applied to cure breast cancer, it still remains one of the leading causes of female deaths worldwide. Since chemotherapeutic drugs have severe side effects and are responsible for development of drug resistance in cancer cells, gene therapy is now considered as one of the promising options to address the current treatment limitations. Identification of the over-expressed genes accounting for constitutive activation of certain pathways, and their subsequent knockdown with specific small interfering RNAs (siRNAs), could be a powerful tool in inhibiting proliferation and survival of cancer cells. In this study, we delivered siRNAs against mRNA transcripts of over-regulated cell adhesion molecules such as catenin alpha 1 (CTNNA1), catenin beta 1 (CTNNB1), talin-1 (TLN1), vinculin (VCL), paxillin (PXN), and actinin-1 (ACTN1) in human (MCF-7 and MDA-MB-231) and murine (4T1) cell lines as well as in the murine female Balb/c mice model. In order to overcome the barriers of cell permeability and nuclease-mediated degradation, the pH-sensitive carbonate apatite (CA) nanocarrier was used as a delivery vehicle. While targeting CTNNA1, CTNNB1, TLN1, VCL, PXN, and ACTN1 resulted in a reduction of cell viability in MCF-7 and MDA-MB-231 cells, delivery of all these siRNAs via carbonate apatite (CA) nanoparticles successfully reduced the cell viability in 4T1 cells. In 4T1 cells, delivery of CTNNA1, CTNNB1, TLN1, VCL, PXN, and ACTN1 siRNAs with CA caused significant reduction in phosphorylated and total AKT levels. Furthermore, reduced band intensity was observed for phosphorylated and total MAPK upon transfection of 4T1 cells with CTNNA1, CTNNB1, and VCL siRNAs. Intravenous delivery of CTNNA1 siRNA with CA nanoparticles significantly reduced tumor volume in the initial phase of the study, while siRNAs targeting CTNNB1, TLN1, VCL, PXN, and ACTN1 genes significantly decreased the tumor burden at all time points. The tumor weights at the end of the treatments were also notably smaller compared to CA. This successfully demonstrates that targeting these dysregulated genes via RNAi and by using a suitable delivery vehicle such as CA could serve as a promising therapeutic treatment modality for breast cancers.
Keywords: breast cancer; carbonate apatite; gene silencing; nanoparticle; paxillin and actinin-1; siRNA; talin-1; vinculin; α-catenin; β-catenin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
siRNAs Targeting Growth Factor Receptor and Anti-Apoptotic Genes Synergistically Kill Breast Cancer Cells through Inhibition of MAPK and PI-3 Kinase Pathways.Biomedicines. 2018 Jun 22;6(3):73. doi: 10.3390/biomedicines6030073. Biomedicines. 2018. PMID: 29932151 Free PMC article.
-
Carbonate Apatite Nanoparticles-Facilitated Intracellular Delivery of siRNA(s) Targeting Calcium Ion Channels Efficiently Kills Breast Cancer Cells.Toxics. 2018 Jun 26;6(3):34. doi: 10.3390/toxics6030034. Toxics. 2018. PMID: 29949888 Free PMC article.
-
Delivery of siRNAs Against Selective Ion Channels and Transporter Genes Using Hyaluronic Acid-coupled Carbonate Apatite Nanoparticles Synergistically Inhibits Growth and Survival of Breast Cancer Cells.Int J Nanomedicine. 2024 Jul 29;19:7709-7727. doi: 10.2147/IJN.S440419. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39099788 Free PMC article.
-
Polyethylenimines for RNAi-mediated gene targeting in vivo and siRNA delivery to the lung.Eur J Pharm Biopharm. 2011 Apr;77(3):438-49. doi: 10.1016/j.ejpb.2010.11.007. Epub 2010 Nov 18. Eur J Pharm Biopharm. 2011. PMID: 21093588 Review.
-
Gene silencing through RNA interference (RNAi) in vivo: strategies based on the direct application of siRNAs.J Biotechnol. 2006 Jun 25;124(1):12-25. doi: 10.1016/j.jbiotec.2005.12.003. Epub 2006 Jan 18. J Biotechnol. 2006. PMID: 16413079 Review.
Cited by
-
Optimization and Formulation of Nanostructured and Self-Assembled Caseinate Micelles for Enhanced Cytotoxic Effects of Paclitaxel on Breast Cancer Cells.Pharmaceutics. 2020 Oct 18;12(10):984. doi: 10.3390/pharmaceutics12100984. Pharmaceutics. 2020. PMID: 33080962 Free PMC article.
-
The Role of CTNNA1 in Malignancies: An Updated Review.J Cancer. 2023 Jan 1;14(2):219-230. doi: 10.7150/jca.79236. eCollection 2023. J Cancer. 2023. PMID: 36741258 Free PMC article. Review.
-
The Role of Paxillin Aberrant Expression in Cancer and Its Potential as a Target for Cancer Therapy.Int J Mol Sci. 2023 May 4;24(9):8245. doi: 10.3390/ijms24098245. Int J Mol Sci. 2023. PMID: 37175948 Free PMC article. Review.
-
PEGylated Strontium Sulfite Nanoparticles with Spontaneously Formed Surface-Embedded Protein Corona Restrict Off-Target Distribution and Accelerate Breast Tumour-Selective Delivery of siRNA.J Funct Biomater. 2022 Nov 1;13(4):211. doi: 10.3390/jfb13040211. J Funct Biomater. 2022. PMID: 36412852 Free PMC article.
-
Effect of plant polysaccharides (Poria cocos and Astragalus polysaccharides) on immune responses and intestinal microbiota of Dabry's sturgeons.Biosci Microbiota Food Health. 2023;42(4):243-253. doi: 10.12938/bmfh.2022-089. Epub 2023 Jun 13. Biosci Microbiota Food Health. 2023. PMID: 37791344 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

