Melatonin Suppresses Microglial Necroptosis by Regulating Deubiquitinating Enzyme A20 After Intracerebral Hemorrhage
- PMID: 31258534
- PMCID: PMC6587666
- DOI: 10.3389/fimmu.2019.01360
Melatonin Suppresses Microglial Necroptosis by Regulating Deubiquitinating Enzyme A20 After Intracerebral Hemorrhage
Abstract
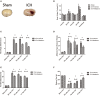
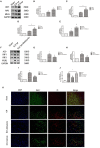
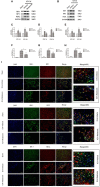
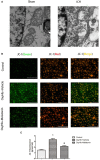
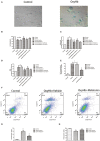
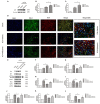
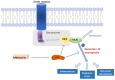
Cell death is deeply involved in pathophysiology of brain injury after intracerebral hemorrhage (ICH). Necroptosis, one of the recently discovered forms of cell death, plays an important role in various diseases, including ICH. Previous studies have suggested that a considerable number of neurons undergoes necroptosis after ICH. However, necroptosis of microglia after ICH has not been reported to date. The present study demonstrated for the first time that necroptosis occurred in the microglia surrounding the hematoma after ICH in C57 mice, and melatonin, a hormone that is predominantly synthesized in and secreted from the pineal gland, exerted a neuroprotective effect by suppressing this process. When we further explored the potential underlying mechanism, we found that melatonin inhibits RIP3-mediated necroptosis by regulating the deubiquitinating enzyme A20 (also known as TNFAIP3) expression after ICH. In summary, we have demonstrated the role of microglial necroptosis in the pathogenesis of ICH. More importantly, A20 was identified as a novel target of melatonin, which opens perspectives for future research.
Keywords: A20; intracerebral hemorrhage (ICH); melatonin; microglia; necroptosis.
Figures
Similar articles
-
A20 Ameliorates Intracerebral Hemorrhage-Induced Inflammatory Injury by Regulating TRAF6 Polyubiquitination.J Immunol. 2017 Jan 15;198(2):820-831. doi: 10.4049/jimmunol.1600334. Epub 2016 Dec 16. J Immunol. 2017. PMID: 27986908 Free PMC article.
-
Necrostatin-1 ameliorates intracerebral hemorrhage-induced brain injury in mice through inhibiting RIP1/RIP3 pathway.Neurochem Res. 2015 Apr;40(4):643-50. doi: 10.1007/s11064-014-1510-0. Epub 2015 Jan 10. Neurochem Res. 2015. PMID: 25576092
-
Perampanel, an AMPAR antagonist, alleviates experimental intracerebral hemorrhage‑induced brain injury via necroptosis and neuroinflammation.Mol Med Rep. 2021 Aug;24(2):544. doi: 10.3892/mmr.2021.12183. Epub 2021 Jun 3. Mol Med Rep. 2021. PMID: 34080030 Free PMC article.
-
Brain injury and repair after intracerebral hemorrhage: The role of microglia and brain-infiltrating macrophages.Neurochem Int. 2021 Jan;142:104923. doi: 10.1016/j.neuint.2020.104923. Epub 2020 Nov 25. Neurochem Int. 2021. PMID: 33248206 Free PMC article. Review.
-
The Critical Role of Erythrolysis and Microglia/Macrophages in Clot Resolution After Intracerebral Hemorrhage: A Review of the Mechanisms and Potential Therapeutic Targets.Cell Mol Neurobiol. 2023 Jan;43(1):59-67. doi: 10.1007/s10571-021-01175-3. Epub 2022 Jan 4. Cell Mol Neurobiol. 2023. PMID: 34981286 Review.
Cited by
-
Neuroprotective Therapies for Spontaneous Intracerebral Hemorrhage.Neurocrit Care. 2021 Dec;35(3):862-886. doi: 10.1007/s12028-021-01311-3. Epub 2021 Aug 2. Neurocrit Care. 2021. PMID: 34341912 Review.
-
Advances in the Study of the Ubiquitin-Editing Enzyme A20.Front Pharmacol. 2022 May 3;13:845262. doi: 10.3389/fphar.2022.845262. eCollection 2022. Front Pharmacol. 2022. PMID: 35592427 Free PMC article. Review.
-
Programmed Cell Deaths and Potential Crosstalk With Blood-Brain Barrier Dysfunction After Hemorrhagic Stroke.Front Cell Neurosci. 2020 Apr 3;14:68. doi: 10.3389/fncel.2020.00068. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32317935 Free PMC article.
-
A prospective cohort study on serum A20 as a prognostic biomarker of aneurysmal subarachnoid hemorrhage.World J Emerg Med. 2023;14(5):360-366. doi: 10.5847/wjem.j.1920-8642.2023.079. World J Emerg Med. 2023. PMID: 37908792 Free PMC article.
-
Aβ oligomers trigger necroptosis-mediated neurodegeneration via microglia activation in Alzheimer's disease.Acta Neuropathol Commun. 2022 Mar 9;10(1):31. doi: 10.1186/s40478-022-01332-9. Acta Neuropathol Commun. 2022. PMID: 35264247 Free PMC article.
References
-
- Hemphill JC, III, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals froma the american heart association/american stroke association. Stroke. (2015) 46:2032–60. 10.1161/STR.0000000000000069 - DOI - PubMed
-
- Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, et al. . Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Global Health. (2013) 1:e259–81. 10.1016/S2214-109X(13)70089-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

