Impact of Superantigen-Producing Bacteria on T Cells from Tonsillar Hyperplasia
- PMID: 31252586
- PMCID: PMC6789895
- DOI: 10.3390/pathogens8030090
Impact of Superantigen-Producing Bacteria on T Cells from Tonsillar Hyperplasia
Abstract
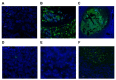
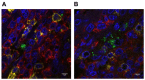
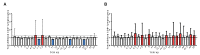
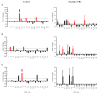
Staphylococcus aureus and Group A Streptococcus (GAS) are common occupants of the tonsils and many strains produce potent exotoxins (mitogens) that directly target T cells, which could be a driver for tonsillar hyperplasia. Tonsil tissues from 41 patients were tested for these bacteria in conjunction with profiling of B and T cells by flow cytometry. S. aureus and GAS were detected in tonsil tissue from 44% and 7%, respectively, of patients by bacteriological culture; immuno-histology showed bacteria in close proximity to both B and T lymphocytes. The presence of tonsillar S. aureus did not alter B or T cell populations, whereas peripheral blood mucosal-associated invariant T (MAIT) cells were significantly increased in S. aureus culture positive individuals (p < 0.006). Alterations of tonsil CD4+ TCR Vβ family members relative to peripheral blood were evident in 29 patients. Three patients had strong TCR Vβ skewing indicative of recent exposure to superantigens, their tonsils contained mitogenic bacteria, and supernatants from these bacteria were used to partially recapitulate the skewing profile in vitro, supporting the notion that superantigens can target tonsillar T cells in situ. Tonsils are a reservoir for superantigen-producing bacteria with the capacity to alter the composition and function of key immune cells.
Keywords: Group A Streptococcus; Staphylococcus aureus; Streptococcus pyogenes; TCR Vβ; mucosal-associated invariant T cells; obstructive sleep apnea; recurrent tonsillitis; superantigen; tonsillar hyperplasia.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
A potential role for staphylococcal and streptococcal superantigens in driving skewing of TCR Vβ subsets in tonsillar hyperplasia.Med Microbiol Immunol. 2017 Aug;206(4):337-346. doi: 10.1007/s00430-017-0510-5. Epub 2017 May 4. Med Microbiol Immunol. 2017. PMID: 28474248
-
The role of Haemophilus influenzae in the pathogenesis of tonsillar hypertrophy in children.Laryngoscope. 1988 Oct;98(10):1055-60. doi: 10.1288/00005537-198810000-00006. Laryngoscope. 1988. PMID: 3262802
-
Tonsillar lymphocyte subsets in recurrent acute tonsillitis and tonsillar hypertrophy.Int J Pediatr Otorhinolaryngol. 1998 Feb;43(1):33-9. doi: 10.1016/s0165-5876(97)00148-1. Int J Pediatr Otorhinolaryngol. 1998. PMID: 9596368
-
Bacteriology of the tonsil core in recurrent tonsillitis and tonsillar hyperplasia--a short review.Acta Otolaryngol Suppl. 2000;543:206-8. doi: 10.1080/000164800454404. Acta Otolaryngol Suppl. 2000. PMID: 10909021 Review.
-
Peritonsillar abscess: clinical aspects of microbiology, risk factors, and the association with parapharyngeal abscess.Dan Med J. 2017 Mar;64(3):B5333. Dan Med J. 2017. PMID: 28260599 Review.
Cited by
-
Mucosa-Associated Invariant T Cell Hypersensitivity to Staphylococcus aureus Leukocidin ED and Its Modulation by Activation.J Immunol. 2022 Mar 1;208(5):1170-1179. doi: 10.4049/jimmunol.2100912. Epub 2022 Feb 9. J Immunol. 2022. PMID: 35140134 Free PMC article.
-
Sleep Disordered Breathing and Recurrent Tonsillitis Are Associated With Polymicrobial Bacterial Biofilm Infections Suggesting a Role for Anti-Biofilm Therapies.Front Cell Infect Microbiol. 2022 Feb 28;12:831887. doi: 10.3389/fcimb.2022.831887. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35295756 Free PMC article.
-
Oral Dysbiosis and Autoimmunity: From Local Periodontal Responses to an Imbalanced Systemic Immunity. A Review.Front Immunol. 2020 Dec 8;11:591255. doi: 10.3389/fimmu.2020.591255. eCollection 2020. Front Immunol. 2020. PMID: 33363538 Free PMC article. Review.
-
Defining T Cell Subsets in Human Tonsils Using ChipCytometry.J Immunol. 2021 Jun 15;206(12):3073-3082. doi: 10.4049/jimmunol.2100063. Epub 2021 Jun 7. J Immunol. 2021. PMID: 34099545 Free PMC article.
-
A subset of follicular helper-like MAIT cells can provide B cell help and support antibody production in the mucosa.Sci Immunol. 2022 Jan 14;7(67):eabe8931. doi: 10.1126/sciimmunol.abe8931. Epub 2022 Jan 14. Sci Immunol. 2022. PMID: 35030034 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

