Imaging fibrosis in inflammatory diseases: targeting the exposed extracellular matrix
- PMID: 31244929
- PMCID: PMC6568181
- DOI: 10.7150/thno.28892
Imaging fibrosis in inflammatory diseases: targeting the exposed extracellular matrix
Abstract
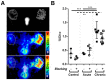
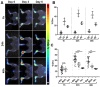
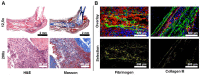
In a variety of diseases, from benign to life-threatening ones, inflammation plays a major role. Monitoring the intensity and extent of a multifaceted inflammatory process has become a cornerstone in diagnostics and therapy monitoring. However, the current tools lack the ability to provide insight into one of its most crucial aspects, namely, the alteration of the extracellular matrix (ECM). Using a radiolabeled platelet glycoprotein VI-based ECM-targeting fusion protein (GPVI-Fc), we investigated how binding of GPVI-Fc on fibrous tissue could uncover the progression of several inflammatory disease models at different stages (rheumatoid arthritis, cutaneous delayed-type hypersensitivity, lung inflammation and experimental autoimmune encephalomyelitis). Methods: The fusion protein GPVI-Fc was covalently linked to 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA) and subsequently labeled with 64Cu. We analyzed noninvasively in vivo64Cu-GPVI-Fc accumulation in murine cutaneous delayed-type hypersensitivity, anti-glucose-6-phosphate isomerase serum-induced rheumatoid arthritis, lipopolysaccharide-induced lung inflammation and an experimental autoimmune encephalomyelitis model. Static and dynamic Positron Emission Tomography (PET) of the radiotracer distribution was performed in vivo, with ex vivo autoradiography confirmation, yielding quantitative accumulation and a distribution map of 64Cu-GPVI-Fc. Ex vivo tissue histological staining was performed on harvested samples to highlight the fusion protein binding to collagen I, II and III, fibronectin and fibrinogen as well as the morphology of excised tissue. Results:64Cu-GPVI-Fc showed a several-fold increased uptake in inflamed tissue compared to control tissue, particularly in the RA model, with a peak 24 h after radiotracer injection of up to half the injected dose. Blocking and isotype control experiments indicated a target-driven accumulation of the radiotracer in the case of chronic inflammation. Histological analysis confirmed a prolonged accumulation at the inflammation site, with a pronounced colocalization with the different components of the ECM (collagen III and fibronectin notably). Binding of the fusion protein appeared to be specific to the ECM but unspecific to particular components. Conclusion: Imaging of 64Cu-GPVI-Fc accumulation in the ECM matrix appears to be a promising candidate for monitoring chronic inflammation. By binding to exposed fibrous tissue (collagen, fibronectin, etc.) after extravasation, a new insight is provided into the fibrotic events resulting from a prolonged inflammatory state.
Keywords: fibrosis; glycoprotein VI; positron emission tomography.
Conflict of interest statement
Competing interests: M. Gawaz is a shareholder of advanceCOR GmbH.
Figures
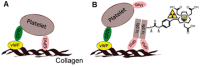


Similar articles
-
Radiolabeled GPVI-Fc for PET Imaging of Multiple Extracellular Matrix Fibers: A New Look into Pulmonary Fibrosis Progression.J Nucl Med. 2023 Jun;64(6):940-945. doi: 10.2967/jnumed.122.264552. Epub 2023 Jan 26. J Nucl Med. 2023. PMID: 36702555 Free PMC article.
-
Positron emission tomography/computed tomographic and magnetic resonance imaging in a murine model of progressive atherosclerosis using (64)Cu-labeled glycoprotein VI-Fc.Circ Cardiovasc Imaging. 2013 Nov;6(6):957-64. doi: 10.1161/CIRCIMAGING.113.000488. Epub 2013 Oct 9. Circ Cardiovasc Imaging. 2013. PMID: 24107491
-
64Cu-DOTA as a surrogate positron analog of Gd-DOTA for cardiac fibrosis detection with PET: pharmacokinetic study in a rat model of chronic MI.Nucl Med Commun. 2016 Feb;37(2):188-96. doi: 10.1097/MNM.0000000000000417. Nucl Med Commun. 2016. PMID: 26488428 Free PMC article.
-
Implications of glycoprotein VI for theranostics.Thromb Haemost. 2014 Jul 3;112(1):26-31. doi: 10.1160/TH13-09-0756. Epub 2014 Feb 20. Thromb Haemost. 2014. PMID: 24553806 Review.
-
Focusing on plasma glycoprotein VI.Thromb Haemost. 2012 Apr;107(4):648-55. doi: 10.1160/TH11-10-0745. Epub 2012 Jan 25. Thromb Haemost. 2012. PMID: 22274761 Review.
Cited by
-
Rheumatoid cachexia: the underappreciated role of myoblast, macrophage and fibroblast interplay in the skeletal muscle niche.J Biomed Sci. 2021 Mar 3;28(1):15. doi: 10.1186/s12929-021-00714-w. J Biomed Sci. 2021. PMID: 33658022 Free PMC article. Review.
-
Location, location, location: how the tissue microenvironment affects inflammation in RA.Nat Rev Rheumatol. 2021 Apr;17(4):195-212. doi: 10.1038/s41584-020-00570-2. Epub 2021 Feb 1. Nat Rev Rheumatol. 2021. PMID: 33526927 Review.
-
The Role of Basement Membranes in Cerebral Amyloid Angiopathy.Front Physiol. 2020 Nov 25;11:601320. doi: 10.3389/fphys.2020.601320. eCollection 2020. Front Physiol. 2020. PMID: 33329053 Free PMC article. Review.
-
Multi-omics Analysis Reveals Adipose-tumor Crosstalk in Patients with Colorectal Cancer.Cancer Prev Res (Phila). 2020 Oct;13(10):817-828. doi: 10.1158/1940-6207.CAPR-19-0538. Epub 2020 Jul 12. Cancer Prev Res (Phila). 2020. PMID: 32655010 Free PMC article.
-
Extracellular vesicles from human umbilical cord blood plasma modulate interleukin-2 signaling of T cells to ameliorate experimental autoimmune encephalomyelitis.Theranostics. 2020 Apr 6;10(11):5011-5028. doi: 10.7150/thno.42742. eCollection 2020. Theranostics. 2020. PMID: 32308765 Free PMC article.
References
-
- Krane S. Overview: Destruction of the Extracellular Matrix in Rheumatoid Arthritis. Arthritis Res Ther; 1999. p. 1.
-
- Ryu JH, Moua T, Daniels CE, Hartman TE, Yi ES, Utz JP. et al. Idiopathic pulmonary fibrosis: evolving concepts. Mayo Clin Proc. 2014;89:1130–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

