MiR-629 regulates hypoxic pulmonary vascular remodelling by targeting FOXO3 and PERP
- PMID: 31240850
- PMCID: PMC6653446
- DOI: 10.1111/jcmm.14385
MiR-629 regulates hypoxic pulmonary vascular remodelling by targeting FOXO3 and PERP
Abstract
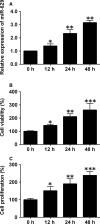
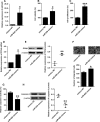
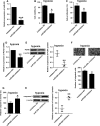
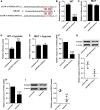
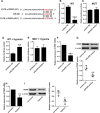
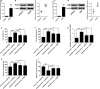
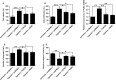
Pulmonary arterial hypertension (PAH) is featured by the increase in pulmonary vascular resistance and pulmonary arterial pressure. Despite that abnormal proliferation and phenotypic changes in human pulmonary artery smooth muscle cells (HPASMCs) contributing to the pathophysiology of PAH, the underlying molecular mechanisms remain unclear. In the present study, we detected the expression of miR-629 in hypoxia-treated HPASMCs and explored the mechanistic role of miR-629 in regulating HPASMC proliferation, migration and apoptosis. Hypoxia time-dependently induced up-regulation of miR-629 and promoted cell viability and proliferation in HPASMCs. Treatment with miR-629 mimics promoted HPASMCs proliferation and migration, but inhibited cell apoptosis; while knockdown of miR-629 suppressed the cell proliferation and migration but promoted cell apoptosis in HPASMCs. The bioinformatics prediction revealed FOXO3 and PERP as downstream targets of miR-629, and miR-629 negatively regulated the expression of FOXO3 and PERP via targeting the 3' untranslated regions. Enforced expression of FOXO3 or PERP attenuated the miR-629 overexpression or hypoxia-induced enhanced effects on HPASMC proliferation and proliferation, and the suppressive effects on HPASMC apoptosis. Furthermore, the expression of miR-629 was up-regulated, and the expression of FOXO3 and PERP mRNA was down-regulated in the plasma from PAH patients when compared to healthy controls. In conclusion, the present study provided evidence regarding the novel role of miR-629 in regulating cell proliferation, migration and apoptosis of HPASMCs during hypoxia.
Keywords: FOXO3; PAH; PERP; PHASMCs; hypoxia; miR-629.
© 2019 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
The authors declare that there are no conflict of interest.
Figures

Similar articles
-
MiR-19a modulates hypoxia-mediated cell proliferation and migration via repressing PTEN in human pulmonary arterial smooth muscle.Life Sci. 2019 Dec 15;239:116928. doi: 10.1016/j.lfs.2019.116928. Epub 2019 Nov 1. Life Sci. 2019. PMID: 31682848
-
MiR-23a regulates the proliferation and migration of human pulmonary artery smooth muscle cells (HPASMCs) through targeting BMPR2/Smad1 signaling.Biomed Pharmacother. 2018 Jul;103:1279-1286. doi: 10.1016/j.biopha.2018.04.172. Epub 2018 May 7. Biomed Pharmacother. 2018. PMID: 29864909
-
CircST6GAL1 knockdown alleviates pulmonary arterial hypertension by regulating miR-509-5p/multiple C2 and transmembrane domain containing 2 axis.Clin Respir J. 2024 May;18(5):e13771. doi: 10.1111/crj.13771. Clin Respir J. 2024. PMID: 38747117 Free PMC article.
-
[Advances in molecular mechanism of vascular remodeling in pulmonary arterial hypertension].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2019 May 25;48(1):102-110. doi: 10.3785/j.issn.1008-9292.2019.02.15. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2019. PMID: 31102364 Free PMC article. Review. Chinese.
-
MicroRNAs in pulmonary arterial remodeling.Cell Mol Life Sci. 2013 Dec;70(23):4479-94. doi: 10.1007/s00018-013-1382-5. Epub 2013 Jun 6. Cell Mol Life Sci. 2013. PMID: 23739951 Free PMC article. Review.
Cited by
-
Bioactivities and mechanisms of natural medicines in the management of pulmonary arterial hypertension.Chin Med. 2022 Jan 15;17(1):13. doi: 10.1186/s13020-022-00568-w. Chin Med. 2022. PMID: 35033157 Free PMC article. Review.
-
Expression Profiles of Circular RNA in Aortic Vascular Tissues of Spontaneously Hypertensive Rats.Front Cardiovasc Med. 2021 Dec 20;8:814402. doi: 10.3389/fcvm.2021.814402. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34988135 Free PMC article.
-
The roles of microRNAs played in lung diseases via regulating cell apoptosis.Mol Cell Biochem. 2021 Dec;476(12):4265-4275. doi: 10.1007/s11010-021-04242-x. Epub 2021 Aug 16. Mol Cell Biochem. 2021. PMID: 34398353 Review.
-
Circulating microRNAs and cardiomyocyte proliferation in heart failure patients related to 10 years survival.ESC Heart Fail. 2023 Dec;10(6):3559-3572. doi: 10.1002/ehf2.14516. Epub 2023 Sep 26. ESC Heart Fail. 2023. PMID: 37752740 Free PMC article.
-
Decrease in LINC00963 attenuates the progression of pulmonary arterial hypertension via microRNA-328-3p/profilin 1 axis.J Clin Lab Anal. 2022 May;36(5):e24383. doi: 10.1002/jcla.24383. Epub 2022 Mar 29. J Clin Lab Anal. 2022. PMID: 35349725 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

