The role of sleep and wakefulness in myelin plasticity
- PMID: 31237382
- PMCID: PMC6771952
- DOI: 10.1002/glia.23667
The role of sleep and wakefulness in myelin plasticity
Abstract
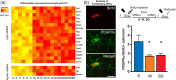
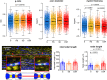
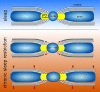
Myelin plasticity is gaining increasing recognition as an essential partner to synaptic plasticity, which mediates experience-dependent brain structure and function. However, how neural activity induces adaptive myelination and which mechanisms are involved remain open questions. More than two decades of transcriptomic studies in rodents have revealed that hundreds of brain transcripts change their expression in relation to the sleep-wake cycle. These studies consistently report upregulation of myelin-related genes during sleep, suggesting that sleep represents a window of opportunity during which myelination occurs. In this review, we summarize recent molecular and morphological studies detailing the dependence of myelin dynamics after sleep, wake, and chronic sleep loss, a condition that can affect myelin substantially. We present novel data about the effects of sleep loss on the node of Ranvier length and provide a hypothetical mechanism through which myelin changes in response to sleep loss. Finally, we discuss the current findings in humans, which appear to confirm the important role of sleep in promoting white matter integrity.
Keywords: brain; myelin; oligodendrocyte; sleep deprivation; white matter.
© 2019 The Authors. Glia published by Wiley Periodicals, Inc.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
Similar articles
-
Axoglial interactions in myelin plasticity: Evaluating the relationship between neuronal activity and oligodendrocyte dynamics.Glia. 2019 Nov;67(11):2038-2049. doi: 10.1002/glia.23629. Epub 2019 Apr 30. Glia. 2019. PMID: 31038804 Review.
-
Myelin plasticity in the central nervous system.Neuropharmacology. 2016 Nov;110(Pt B):563-573. doi: 10.1016/j.neuropharm.2015.08.001. Epub 2015 Aug 15. Neuropharmacology. 2016. PMID: 26282119 Review.
-
Building a (w)rapport between neurons and oligodendroglia: Reciprocal interactions underlying adaptive myelination.Neuron. 2021 Apr 21;109(8):1258-1273. doi: 10.1016/j.neuron.2021.02.003. Epub 2021 Feb 22. Neuron. 2021. PMID: 33621477 Free PMC article. Review.
-
Periods of synchronized myelin changes shape brain function and plasticity.Nat Neurosci. 2021 Nov;24(11):1508-1521. doi: 10.1038/s41593-021-00917-2. Epub 2021 Oct 28. Nat Neurosci. 2021. PMID: 34711959 Review.
-
On Myelinated Axon Plasticity and Neuronal Circuit Formation and Function.J Neurosci. 2017 Oct 18;37(42):10023-10034. doi: 10.1523/JNEUROSCI.3185-16.2017. J Neurosci. 2017. PMID: 29046438 Free PMC article. Review.
Cited by
-
Melatonin signalling in Schwann cells during neuroregeneration.Front Cell Dev Biol. 2022 Oct 10;10:999322. doi: 10.3389/fcell.2022.999322. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36299487 Free PMC article. Review.
-
Individual differences in slow wave sleep architecture relate to variation in white matter microstructure across adulthood.Front Aging Neurosci. 2022 Aug 25;14:745014. doi: 10.3389/fnagi.2022.745014. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36092806 Free PMC article.
-
In vitro cell models merging circadian rhythms and brain waves for personalized neuromedicine.iScience. 2022 Nov 2;25(12):105477. doi: 10.1016/j.isci.2022.105477. eCollection 2022 Dec 22. iScience. 2022. PMID: 36387022 Free PMC article. Review.
-
Effects of Visual Deprivation on Remodeling of Nodes of Ranvier in Optic Nerve.eNeuro. 2022 Nov 10;9(6):ENEURO.0194-22.2022. doi: 10.1523/ENEURO.0194-22.2022. Print 2022 Nov-Dec. eNeuro. 2022. PMID: 36302632 Free PMC article.
-
Nighttime Sleep Characteristics and White Matter Integrity in Young Adults.Nat Sci Sleep. 2022 Aug 6;14:1363-1373. doi: 10.2147/NSS.S360311. eCollection 2022. Nat Sci Sleep. 2022. PMID: 35965887 Free PMC article.
References
-
- Aggarwal, S. , Yurlova, L. , & Simons, M. (2011). Central nervous system myelin: Structure, synthesis and assembly. Trends in Cell Biology, 21, 585–593. - PubMed
-
- Amorim, L. , Magalhães, R. , Coelho, A. , Moreira, P. S. , Portugal‐Nunes, C. , Castanho, T. C. , … Santos, N. C. (2018). Poor sleep quality associates with decreased functional and structural brain connectivity in normative aging: A MRI multimodal approach. Frontiers in Aging Neuroscience, 10, 375. - PMC - PubMed
-
- Armati, P. , & Mathey, E. (2010). The biology of Oligodendrocytes. Cambridge, UK: Cambridge University Press.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

