Study on efficacy and safety of Tong-luo Qu-tong plaster treatment for knee osteoarthritis: study protocol for a randomized, double-blind, parallel positive controlled, multi-center clinical trial
- PMID: 31234919
- PMCID: PMC6591844
- DOI: 10.1186/s13063-019-3481-6
Study on efficacy and safety of Tong-luo Qu-tong plaster treatment for knee osteoarthritis: study protocol for a randomized, double-blind, parallel positive controlled, multi-center clinical trial
Abstract
Background: Knee osteoarthritis (KOA) is a common chronic musculoskeletal disorder that seriously affects quality of life. Patients with KOA frequently develop one or more of the following typical symptoms: joint pain, stiffness, joint friction noise and impaired functionality. Traditional Chinese medicine (TCM) has been shown to have a superior effect and a particular advantage in the treatment of KOA; among TCM, the Tong-luo Qu-tong plaster is the convenient and most commonly used method in China to improve symptoms including pain, stiffness and limited mobility in patients with KOA, as it causes few adverse effects. But there is a lack of high-quality clinical evidences to support the therapeutic effect that Chinese adhesive plaster can have in relieving pain and stiffness. The purpose of this study will be to evaluate the efficacy and safety of Tong-luo Qu-tong plaster in patients with KOA.
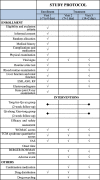
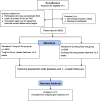
Methods/design: This study will be a randomized, double-blind, parallel positive controlled, multi-center clinical trial, a non-inferiority trial design was adopted. A total of 2000 participants older than 40 years, with KOA, will be randomly allocated into an experimental group (n = 1500) and a control group (n = 500). All participants will receive a conventional conservative treatment lasting for 14 days as two courses, once daily. Tong-luo Qu-tong plaster will be administered externally to participants in the experimental group, while the control group will receive a Qi-zheng Xiao-tong plaster. The outcome of the total Western Ontario and McMaster Universities Arthritis Index scores, TCM syndrome quantitative score and visual analog scale scores will be measured during the assessment visits (baseline and 1-week and 2-week follow up). In addition, adverse events related to clinical symptoms and signs and results of laboratory tests will be documented during the clinical trials.
Discussion: This study will provide reliable evidence of the effectiveness and safety of Tong-luo Qutong plaster in patients with KOA. If the results are favorable, it is expected that the patients with KOA will benefit from this study, many patients may have a good alternative treatment for KOA.
Trial registration: ClinicalTrials.gov, ID: NCT03309501 . Registered on 8 November 2017.
Keywords: Clinical trials; Knee osteoarthritis; Protocol; Randomized; Tong-luo Qu-tong plaster.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Golden plaster for pain therapy in patients with knee osteoarthritis: study protocol for a multicenter randomized, double-blind, placebo-controlled trial.Trials. 2013 Nov 13;14:383. doi: 10.1186/1745-6215-14-383. Trials. 2013. PMID: 24220504 Free PMC article. Clinical Trial.
-
Efficacy and safety of traditional Chinese medicine rehabilitation program in the treatment of knee osteoarthritis: a randomized controlled trial protocol.Ann Palliat Med. 2021 Jun;10(6):6909-6918. doi: 10.21037/apm-21-1179. Ann Palliat Med. 2021. PMID: 34237988
-
Huo-Luo-Xiao-Ling (HLXL)-Dan, a Traditional Chinese Medicine, for patients with osteoarthritis of the knee: a multi-site, randomized, double-blind, placebo-controlled phase II clinical trial.Osteoarthritis Cartilage. 2015 Dec;23(12):2102-2108. doi: 10.1016/j.joca.2015.06.007. Epub 2015 Jun 20. Osteoarthritis Cartilage. 2015. PMID: 26099553 Free PMC article. Clinical Trial.
-
Oral traditional Chinese medication for adhesive small bowel obstruction.Cochrane Database Syst Rev. 2012 May 16;(5):CD008836. doi: 10.1002/14651858.CD008836.pub2. Cochrane Database Syst Rev. 2012. PMID: 22592734 Review.
-
Research Progress in Single-herb Chinese Medicine and Compound Medicine for Knee Osteoarthritis.Comb Chem High Throughput Screen. 2024;27(15):2180-2186. doi: 10.2174/0113862073264850231116055745. Comb Chem High Throughput Screen. 2024. PMID: 38305402 Free PMC article. Review.
Cited by
-
Network Pharmacology Approach to Uncover the Mechanism Governing the Effect of Radix Achyranthis Bidentatae on Osteoarthritis.BMC Complement Med Ther. 2020 Apr 21;20(1):121. doi: 10.1186/s12906-020-02909-4. BMC Complement Med Ther. 2020. PMID: 32316966 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources