FSH/LH-Dependent Upregulation of Ahr in Murine Granulosa Cells Is Controlled by PKA Signaling and Involves Epigenetic Regulation
- PMID: 31234584
- PMCID: PMC6627912
- DOI: 10.3390/ijms20123068
FSH/LH-Dependent Upregulation of Ahr in Murine Granulosa Cells Is Controlled by PKA Signaling and Involves Epigenetic Regulation
Abstract
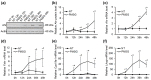
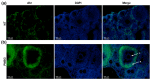
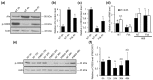
The aryl hydrocarbon receptor (Ahr) is a ligand-activated transcription factor primarily known for its toxicological functions. Recent studies have established its importance in many physiological processes including female reproduction, although there is limited data about the precise mechanisms how Ahr itself is regulated during ovarian follicle maturation. This study describes the expression of Ahr in ovarian granulosa cells (GCs) of immature mice in a gonadotropin-dependent manner. We show that Ahr upregulation in vivo requires both follicle stimulating hormone (FSH) and luteinizing hormone (LH) activities. FSH alone increased Ahr mRNA, but had no effect on Ahr protein level, implicating a possible LH-dependent post-transcriptional regulation. Also, the increase in Ahr protein is specific to large antral follicles in induced follicle maturation. We show that Ahr expression in GCs of mid-phase follicular maturation is downregulated by protein kinase A (PKA) signaling and activation of Ahr promoter is regulated by chromatin remodeling.
Keywords: aryl hydrocarbon receptor (AhR); chromatin remodeling; follicle-stimulating hormone (FSH); luteinizing hormone (LH); post-transcriptional regulation; protein kinase A (PKA).
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Transcriptional repression of the Ahr gene by LHCGR signaling in preovulatory granulosa cells is controlled by chromatin accessibility.Mol Cell Endocrinol. 2014 Jan 25;382(1):292-301. doi: 10.1016/j.mce.2013.10.011. Epub 2013 Oct 18. Mol Cell Endocrinol. 2014. PMID: 24145128
-
Lhcgr expression in granulosa cells: roles for PKA-phosphorylated β-catenin, TCF3, and FOXO1.Mol Endocrinol. 2013 Aug;27(8):1295-310. doi: 10.1210/me.2013-1025. Epub 2013 Jun 10. Mol Endocrinol. 2013. PMID: 23754802 Free PMC article.
-
FSH and its second messenger cAMP stimulate the transcription of human anti-Müllerian hormone in cultured granulosa cells.Mol Endocrinol. 2011 Apr;25(4):645-55. doi: 10.1210/me.2010-0297. Epub 2011 Feb 17. Mol Endocrinol. 2011. PMID: 21330407 Free PMC article.
-
ERβ Regulation of Gonadotropin Responses during Folliculogenesis.Int J Mol Sci. 2021 Sep 26;22(19):10348. doi: 10.3390/ijms221910348. Int J Mol Sci. 2021. PMID: 34638689 Free PMC article. Review.
-
Ovarian follicular and luteal physiology.Int Rev Physiol. 1980;22:117-201. Int Rev Physiol. 1980. PMID: 6248477 Review.
Cited by
-
High levels of follicular fluid testosterone could impair oocyte developmental competency via affecting aryl hydrocarbon receptor pathway in PCOS patients.BMC Mol Cell Biol. 2022 Nov 11;23(1):47. doi: 10.1186/s12860-022-00449-y. BMC Mol Cell Biol. 2022. PMID: 36368943 Free PMC article.
-
Triazole pesticides exposure impaired steroidogenesis associated to an increase in AHR and CAR expression in testis and altered sperm parameters in chicken.Toxicol Rep. 2023 Mar 21;10:409-427. doi: 10.1016/j.toxrep.2023.03.005. eCollection 2023. Toxicol Rep. 2023. PMID: 37025555 Free PMC article.
-
Anti-Aging Physiological Roles of Aryl Hydrocarbon Receptor and Its Dietary Regulators.Int J Mol Sci. 2020 Dec 31;22(1):374. doi: 10.3390/ijms22010374. Int J Mol Sci. 2020. PMID: 33396477 Free PMC article. Review.
-
Pathophysiological Changes in Female Rats with Estrous Cycle Disorder Induced by Long-Term Heat Stress.Biomed Res Int. 2020 Jun 17;2020:4701563. doi: 10.1155/2020/4701563. eCollection 2020. Biomed Res Int. 2020. PMID: 32685488 Free PMC article.
-
Assisted reproductive technology and interactions between serum basal FSH/LH and ovarian sensitivity index.Front Endocrinol (Lausanne). 2023 May 3;14:1086924. doi: 10.3389/fendo.2023.1086924. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37206442 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

