Acute corneal injury in rabbits following nitrogen mustard ocular exposure
- PMID: 31233733
- PMCID: PMC6754274
- DOI: 10.1016/j.yexmp.2019.104275
Acute corneal injury in rabbits following nitrogen mustard ocular exposure
Abstract
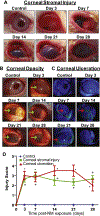
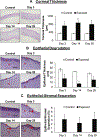
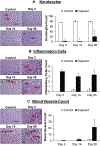
Sulfur mustard (SM), a potent vesicating chemical warfare agent, and its analog nitrogen mustard (NM), are both strong bi-functional alkylating agents. Eyes, skin, and the respiratory system are the main targets of SM and NM exposure; however, ocular tissue is most sensitive, resulting in severe ocular injury. The mechanism of ocular injury from vesicating agents' exposure is not completely understood. To understand the injury mechanism from exposure to vesicating agents, NM has been previously employed in our toxicity studies on primary human corneal epithelial cells and ex vivo rabbit cornea organ culture model. In the current study, corneal toxicity from NM ocular exposure (1%) was analyzed for up to 28 days post-exposure in New Zealand White male rabbits to develop an acute corneal injury model. NM exposure led to conjunctival and eyelid swelling within a few hours after exposure, in addition to significant corneal opacity and ulceration. An increase in total corneal thickness and epithelial degradation was observed starting at day 3 post-NM exposure, which was maximal at day 14 post-exposure and did not resolve until 28 days post-exposure. There was an NM-induced increase in the number of blood vessels and inflammatory cells, and a decrease in keratocytes in the corneal stroma. NM exposure resulted in increased expression levels of cyclooxygenase-2, Interleukin-8, vascular endothelial growth factor and Matrix Metalloproteinase 9 indicating their involvement in NM-induced corneal injury. These clinical, biological, and molecular markers could be useful for the evaluation of acute corneal injury and to screen for therapies against NM- and SM-induced ocular injury.
Keywords: Corneal injury; Inflammation; Mustard; Nitrogen mustard; Sulfur mustard; Vesicant.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTEREST
The authors declare no conflict of interest.
Figures

Similar articles
-
Nitrogen Mustard-Induced Corneal Injury Involves DNA Damage and Pathways Related to Inflammation, Epithelial-Stromal Separation, and Neovascularization.Cornea. 2016 Feb;35(2):257-66. doi: 10.1097/ICO.0000000000000685. Cornea. 2016. PMID: 26555588 Free PMC article.
-
Nitrogen Mustard-Induced Ex Vivo Human Cornea Injury Model and Therapeutic Intervention by Dexamethasone.J Pharmacol Exp Ther. 2024 Jan 17;388(2):484-494. doi: 10.1124/jpet.123.001760. J Pharmacol Exp Ther. 2024. PMID: 37474260 Free PMC article.
-
Pathophysiology and inflammatory biomarkers of sulfur mustard-induced corneal injury in rabbits.PLoS One. 2021 Oct 12;16(10):e0258503. doi: 10.1371/journal.pone.0258503. eCollection 2021. PLoS One. 2021. PMID: 34637469 Free PMC article.
-
Research models of sulfur mustard- and nitrogen mustard-induced ocular injuries and potential therapeutics.Exp Eye Res. 2022 Oct;223:109209. doi: 10.1016/j.exer.2022.109209. Epub 2022 Aug 9. Exp Eye Res. 2022. PMID: 35961426 Review.
-
Corneal toxicity induced by vesicating agents and effective treatment options.Ann N Y Acad Sci. 2016 Jun;1374(1):193-201. doi: 10.1111/nyas.13121. Epub 2016 Jun 21. Ann N Y Acad Sci. 2016. PMID: 27327041 Free PMC article. Review.
Cited by
-
Dysregulation of the mTOR pathway by mechlorethamine.Toxicology. 2023 Mar 1;486:153434. doi: 10.1016/j.tox.2023.153434. Epub 2023 Jan 26. Toxicology. 2023. PMID: 36708981 Free PMC article.
-
Ocular Surface - Merging Challenges and Opportunities.Transl Vis Sci Technol. 2020 Nov 2;9(12):3. doi: 10.1167/tvst.9.12.3. eCollection 2020 Nov. Transl Vis Sci Technol. 2020. PMID: 33200045 Free PMC article.
-
Insights into mustard gas keratopathy- characterizing corneal layer-specific changes in mice exposed to nitrogen mustard.Exp Eye Res. 2023 Nov;236:109657. doi: 10.1016/j.exer.2023.109657. Epub 2023 Sep 16. Exp Eye Res. 2023. PMID: 37722586 Free PMC article.
-
Mouse Model of Nitrogen Mustard Ocular Surface Injury Characterization and Sphingolipid Signaling.Int J Mol Sci. 2024 Jan 6;25(2):742. doi: 10.3390/ijms25020742. Int J Mol Sci. 2024. PMID: 38255815 Free PMC article.
-
Dexamethasone sodium phosphate loaded nanoparticles for prevention of nitrogen mustard induced corneal injury.Exp Eye Res. 2024 Jun;243:109902. doi: 10.1016/j.exer.2024.109902. Epub 2024 Apr 18. Exp Eye Res. 2024. PMID: 38641196
References
-
- Amir A, et al., 2000. Beneficial effects of topical anti-inflammatory drugs against sulfur mustard-induced ocular lesions in rabbits. J Appl Toxicol. 20 Suppl 1, S109–14. - PubMed
-
- Balali-Mood M, et al., 2011. Delayed toxic effects of sulfur mustard on respiratory tract of Iranian veterans. Hum Exp Toxicol. 30,1141–9. - PubMed
-
- Balali-Mood M, Hefazi M, 2006. Comparison of early and late toxic effects of sulfur mustard in Iranian veterans. Basic Clin Pharmacol Toxicol. 99, 273–82. - PubMed
-
- Banin E, et al., 2003. Injury induced by chemical warfare agents: characterization and treatment of ocular tissues exposed to nitrogen mustard. Invest Ophthalmol Vis Sci. 44, 2966–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

