Gut Microbiota Regulates Mincle Mediated Activation of Lung Dendritic Cells to Protect Against Mycobacterium tuberculosis
- PMID: 31231363
- PMCID: PMC6558411
- DOI: 10.3389/fimmu.2019.01142
Gut Microbiota Regulates Mincle Mediated Activation of Lung Dendritic Cells to Protect Against Mycobacterium tuberculosis
Abstract
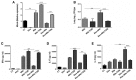
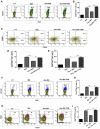
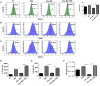
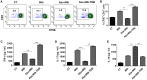
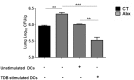
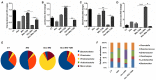
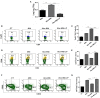
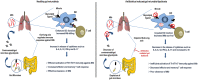
Gut microbial components serve as ligand for various pattern recognition receptors (PRRs) present on immune cells and thereby regulates host immunity. Dendritic cells (DCs) are highly specialized innate cells involved in immune response to Mycobacterium tuberculosis (Mtb) infection. The gut-lung axis is a potential therapeutic target in tuberculosis; however, understanding of the innate immune mechanism underlying the interaction of gut microbiota and lung still remains obscure. We investigated if antibiotics (Abx) induced gut dysbiosis is able to affect the activation of innate receptor, macrophage inducible C-type lectin (mincle) in lungs during Mtb infection. We found that dysbiosis reduced the lung mincle expression with a concomitant increase in Mtb survival. Further, Abx diminished the effector and memory T cell population, while elevating frequency of regulatory T cells (Tregs) in the lungs. Here, we show that dysbiotic mice exhibited low mincle expression on lung DCs. These DCs with impaired phenotype and functions had reduced ability to activate naïve CD4 T cells, and thus unable to restrict Mtb survival. In vivo administration of trehalose-6,6-dibehenate (TDB: mincle ligand) efficiently rescued this immune defect by enhancing lung DCs function and subsequent T cell response. Further, gut microbial profiling revealed augmentation of Lactobacillus upon mincle stimulation in microbiota depleted animals. Accordingly, supplementation with Lactobacillus restored mincle expression on lung DCs along with anti-Mtb response. Our data demonstrate that gut microbiota is crucial to maintain DC-dependent lung immune response against Mtb, mediated by mincle. Abx interrupt this process to induce impaired T cell-response and increased susceptibility to Mtb.
Keywords: T cells; antibiotics; gut-lung axis; lung dendritic cells; mincle; tuberculosis.
Figures


Similar articles
-
Intestinal microbiota disruption limits the isoniazid mediated clearance of Mycobacterium tuberculosis in mice.Eur J Immunol. 2020 Dec;50(12):1976-1987. doi: 10.1002/eji.202048556. Epub 2020 Jul 29. Eur J Immunol. 2020. PMID: 32673409
-
Mincle is not essential for controlling Mycobacterium tuberculosis infection.Immunobiology. 2013 Apr;218(4):506-16. doi: 10.1016/j.imbio.2012.06.005. Epub 2012 Jun 21. Immunobiology. 2013. PMID: 22784441
-
Induction of autophagy through CLEC4E in combination with TLR4: an innovative strategy to restrict the survival of Mycobacterium tuberculosis.Autophagy. 2020 Jun;16(6):1021-1043. doi: 10.1080/15548627.2019.1658436. Epub 2019 Sep 8. Autophagy. 2020. PMID: 31462144 Free PMC article.
-
Mycobacterium tuberculosis, antimicrobials, immunity, and lung-gut microbiota crosstalk: current updates and emerging advances.Ann N Y Acad Sci. 2020 May;1467(1):21-47. doi: 10.1111/nyas.14300. Epub 2020 Jan 28. Ann N Y Acad Sci. 2020. PMID: 31989644 Review.
-
Distinct Strategies Employed by Dendritic Cells and Macrophages in Restricting Mycobacterium tuberculosis Infection: Different Philosophies but Same Desire.Int Rev Immunol. 2016 Sep 2;35(5):386-398. doi: 10.3109/08830185.2015.1015718. Epub 2015 Mar 20. Int Rev Immunol. 2016. PMID: 25793750 Review.
Cited by
-
The Gut Microbiota and Respiratory Diseases: New Evidence.J Immunol Res. 2020 Jul 31;2020:2340670. doi: 10.1155/2020/2340670. eCollection 2020. J Immunol Res. 2020. PMID: 32802893 Free PMC article. Review.
-
Intestinal microbiota and tuberculosis: Insights from Mendelian randomization.Medicine (Baltimore). 2024 Jul 5;103(27):e38762. doi: 10.1097/MD.0000000000038762. Medicine (Baltimore). 2024. PMID: 38968531 Free PMC article.
-
The lung-gut crosstalk in respiratory and inflammatory bowel disease.Front Cell Infect Microbiol. 2023 Aug 23;13:1218565. doi: 10.3389/fcimb.2023.1218565. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37680747 Free PMC article. Review.
-
A murine model of tuberculosis/type 2 diabetes comorbidity for investigating the microbiome, metabolome and associated immune parameters.Animal Model Exp Med. 2021 Mar 23;4(2):181-188. doi: 10.1002/ame2.12159. eCollection 2021 Jun. Animal Model Exp Med. 2021. PMID: 34179725 Free PMC article.
-
Microbiota and Immunity during Respiratory Infections: Lung and Gut Affair.Int J Mol Sci. 2024 Apr 5;25(7):4051. doi: 10.3390/ijms25074051. Int J Mol Sci. 2024. PMID: 38612860 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

