Antileukemia Effects of Notch-Mediated Inhibition of Oncogenic PLK1 in B-Cell Acute Lymphoblastic Leukemia
- PMID: 31227645
- PMCID: PMC6726528
- DOI: 10.1158/1535-7163.MCT-18-0706
Antileukemia Effects of Notch-Mediated Inhibition of Oncogenic PLK1 in B-Cell Acute Lymphoblastic Leukemia
Abstract
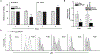
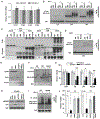
In B-cell acute lymphoblastic leukemia (B-ALL), activation of Notch signaling leads to cell-cycle arrest and apoptosis. We aimed to harness knowledge acquired by understanding a mechanism of Notch-induced cell death to elucidate a therapeutically viable target in B-ALL. To this end, we identified that Notch activation suppresses Polo-like kinase 1 (PLK1) in a B-ALL-specific manner. We identified that PLK1 is expressed in all subsets of B-ALL and is highest in Philadelphia-like (Ph-like) ALL, a high-risk subtype of disease. We biochemically delineated a mechanism of Notch-induced PLK1 downregulation that elucidated stark regulation of p53 in this setting. Our findings identified a novel posttranslational cascade initiated by Notch in which CHFR was activated via PARP1-mediated PARylation, resulting in ubiquitination and degradation of PLK1. This led to hypophosphorylation of MDM2Ser260, culminating in p53 stabilization and upregulation of BAX. shRNA knockdown or pharmacologic inhibition of PLK1 using BI2536 or BI6727 (volasertib) in B-ALL cell lines and patient samples led to p53 stabilization and cell death. These effects were seen in primary human B-ALL samples in vitro and in patient-derived xenograft models in vivo These results highlight PLK1 as a viable therapeutic target in B-ALL. Efficacy of clinically relevant PLK1 inhibitors in B-ALL patient-derived xenograft mouse models suggests that use of these agents may be tailored as an additional therapeutic strategy in future clinical studies.
©2019 American Association for Cancer Research.
Conflict of interest statement
Disclosure of Conflicts of Interest:
The authors declare no conflict of interest.
Figures


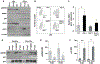

Similar articles
-
In vitro study of the Polo-like kinase 1 inhibitor volasertib in non-small-cell lung cancer reveals a role for the tumor suppressor p53.Mol Oncol. 2019 May;13(5):1196-1213. doi: 10.1002/1878-0261.12477. Epub 2019 Apr 5. Mol Oncol. 2019. PMID: 30859681 Free PMC article.
-
Polo-like Kinase-1 Regulates Myc Stabilization and Activates a Feedforward Circuit Promoting Tumor Cell Survival.Mol Cell. 2016 Nov 3;64(3):493-506. doi: 10.1016/j.molcel.2016.09.016. Epub 2016 Oct 20. Mol Cell. 2016. PMID: 27773673
-
PLK1 is a critical determinant of tumor cell sensitivity to CPT11 and its inhibition enhances the drug antitumor efficacy in squamous cell carcinoma models sensitive and resistant to camptothecins.Oncotarget. 2015 Apr 20;6(11):8736-49. doi: 10.18632/oncotarget.3538. Oncotarget. 2015. PMID: 25826089 Free PMC article.
-
Spotlight on Volasertib: Preclinical and Clinical Evaluation of a Promising Plk1 Inhibitor.Med Res Rev. 2016 Jul;36(4):749-86. doi: 10.1002/med.21392. Epub 2016 May 3. Med Res Rev. 2016. PMID: 27140825 Review.
-
BI_2536--targeting the mitotic kinase Polo-like kinase 1 (Plk1).Recent Results Cancer Res. 2010;184:215-8. doi: 10.1007/978-3-642-01222-8_15. Recent Results Cancer Res. 2010. PMID: 20072841 Review.
Cited by
-
Targeting the NRF2/HO-1 Antioxidant Pathway in FLT3-ITD-Positive AML Enhances Therapy Efficacy.Antioxidants (Basel). 2022 Apr 5;11(4):717. doi: 10.3390/antiox11040717. Antioxidants (Basel). 2022. PMID: 35453402 Free PMC article.
-
MicroRNAs as Modulators of the Immune Response in T-Cell Acute Lymphoblastic Leukemia.Int J Mol Sci. 2022 Jan 13;23(2):829. doi: 10.3390/ijms23020829. Int J Mol Sci. 2022. PMID: 35055013 Free PMC article. Review.
-
The Identification of Stemness-Related Genes in the Risk of Head and Neck Squamous Cell Carcinoma.Front Oncol. 2021 Jun 11;11:688545. doi: 10.3389/fonc.2021.688545. eCollection 2021. Front Oncol. 2021. PMID: 34178686 Free PMC article.
-
Human PARP1 substrates and regulators of its catalytic activity: An updated overview.Front Pharmacol. 2023 Feb 23;14:1137151. doi: 10.3389/fphar.2023.1137151. eCollection 2023. Front Pharmacol. 2023. PMID: 36909172 Free PMC article. Review.
-
The Complexity of the Tumor Microenvironment and Its Role in Acute Lymphoblastic Leukemia: Implications for Therapies.Front Oncol. 2021 May 5;11:673506. doi: 10.3389/fonc.2021.673506. eCollection 2021. Front Oncol. 2021. PMID: 34026651 Free PMC article. Review.
References
-
- Kato M, Manabe A. Treatment and biology of pediatric acute lymphoblastic leukemia. Pediatr Int. 2018;60(1):4–12. - PubMed
-
- Jabbour E, O’Brien S, Konopleva M, Kantarjian H. New insights into the pathophysiology and therapy of adult acute lymphoblastic leukemia. Cancer. 2015;121(15):2517–28. - PubMed
-
- Vrooman LM, Silverman LB. Treatment of Childhood Acute Lymphoblastic Leukemia: Prognostic Factors and Clinical Advances. Current hematologic malignancy reports. 2016;11(5):385–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

