Oral 5-aminosalicylic acid for maintenance of surgically-induced remission in Crohn's disease
- PMID: 31220875
- PMCID: PMC6586553
- DOI: 10.1002/14651858.CD008414.pub3
Oral 5-aminosalicylic acid for maintenance of surgically-induced remission in Crohn's disease
Abstract
Background: Crohn's disease (CD) is a chronic inflammatory disorder that can involve any part of the gastrointestinal tract. 5-Aminosalicylates (5-ASAs) are locally acting, anti-inflammatory compounds that reduce inflammation of the colonic mucosa with release profiles that vary among various commercially available formulations. This updated Cochrane review summarizes current evidence on the use of 5-ASA formulations for maintenance of surgically-induced remission in CD.
Objectives: To assess the efficacy and safety of 5-ASA agents for the maintenance of surgically-induced remission in CD.
Search methods: We searched MEDLINE, Embase, CENTRAL, the Cochrane IBD Group Specialized Register from inception to 16 July 2018. We also searched references, conference abstracts, and trials registers.
Selection criteria: Randomised controlled trials (RCTs) that included participants with CD in remission following surgery and compared 5-ASAs to no treatment, placebo or any other active intervention with duration of at least three months were considered for inclusion.
Data collection and analysis: We used standard methodological procedures expected by Cochrane. The primary outcome was clinical relapse. Secondary outcomes included endoscopic recurrence, radiologic and surgical relapse, adverse events, serious adverse events and withdrawal due to adverse events.
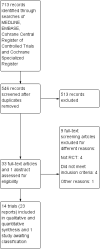
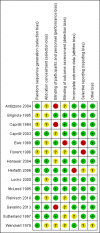
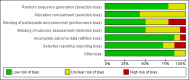
Main results: Fourteen RCTs (1867 participants) were included in the review. Participants (15 to 70 years) were recruited from gastroenterology hospitals and medical clinics in Europe and North America and followed up between 3 and 72 months. The risk of bias was assessed as 'low' in one study, 'unclear' in seven and as 'high' in six.At 12 months, 36% (20/55) of participants in the 5-ASA group experienced clinical relapse compared to 51% (28/55) in the no treatment control group (RR 0.71, 95% CI 0.46 to 1.10; low certainty evidence). Moderate certainty evidence suggests that 5-ASAs are more effective for preventing clinical relapse than placebo. During a follow-up period of 12 to 72 months, 36% (131/361) of 5-ASA participants relapsed compared to 43% (160/369) of placebo participants (RR 0.83, 95% CI 0.72 to 0.96; I² = 0%; moderate certainty evidence). At 12 months, 17% (17/101) of the 4 g/day mesalamine group relapsed compared to 26% (27/105) of the 2.4 g/day group (RR 0.65, 95% CI 0.38 to 1.13; moderate certainty evidence). There was no evidence of a difference in clinical relapse rates when 5-ASA compounds were compared to purine antimetabolites. At 24 months, 61% (103/170) of mesalamine participants relapsed compared to 67% (119/177) of azathioprine participants (RR 0.90, 95% CI 0.76 to 1.07; I² = 28%; low certainty evidence). During 24 months, 50% (9/18) of 5-ASA participants had clinical relapse compared to 13% (2/16) of adalimumab participants (RR 4.0, 95% CI 1.01 to 15.84; low certainty evidence). The effects of sulphasalazine compared to placebo on clinical relapse rate is uncertain. After 18 to 36 months, 66% (95/143) of participants treated with sulphasalazine relapsed compared to 71% (110/155) in the placebo group (RR 0.88, 95% CI 0.56 to 1.38; I² = 38%; low certainty evidence).The effect of 5-ASA drugs on safety was uncertain. During 24 months follow-up, 4% (2/55) of 5-ASA participants experienced adverse events compared to none (0/55) in the no treatment control group (RR 5.00, 95% CI 0.25 to 101.81; very low certainty evidence). An equal proportion of 5-ASA participants (10%; 23/241) and placebo (9%; 20/225) groups experienced an adverse event during a follow-up of 3 to 72 months (RR 1.07, 95% CI 0.60 to 1.91; I² = 0%; low certainty evidence). Adverse event rates were similar in the 5-ASA and purine analogues groups. However, serious adverse events and withdrawals due to adverse events were more common in participants who received purine analogues than 5-ASA. At 52 weeks to 24 months, 52% (107/207) of 5-ASA participants had an adverse event compared to 47% (102/218) of purine analogue participants (RR 1.11, 95% CI 0.97 to 1.27, I² = 0%; low certainty evidence). Four per cent (6/152) of 5-ASA participants had a serious adverse event compared to 17% (27/159) of purine analogue participants (RR 0.30, 95% CI 0.11 to 0.80; very low certainty evidence). Eight per cent (17/207) of 5-ASA participants withdrew due to an adverse event compared to 19% (42/218) of purine analogue participants (RR 0.48, 95% CI 0.28 to 0.83; low certainty evidence). Adverse event rates were similar in high and low dose mesalamine participants. After 12 months, 2% (2/101) of 4 g/day mesalamine participants had an adverse event compared to 2% (2/105) of 2.4 g/day participants (RR 1.04, 95% CI 0.15 to 7.24; low certainty evidence). The proportion of participants who experienced adverse events over a 24 month follow-up in the mesalamine group was 78% (14/18) compared to 69% (11/16) of adalimumab participants (RR 1.13, 95% CI 0.75 to 1.71; very low certainty evidence). None (0/32) of the sulphasalazine participants had an adverse event at 18 months follow-up compared to 3% (1/34) of the placebo group (RR 0.35, 95% CI 0.01 to 8.38; very low certainty evidence). Commonly reported adverse events in the included studies were diarrhoea, nausea, increased liver function tests, pancreatitis, and abdominal pain.
Authors' conclusions: 5-ASA preparations are superior to placebo for the maintenance of surgically-induced clinical remission in patients with CD (moderate certainty). The number needed to treat to prevent one relapse was 13 patients. The evidence for endoscopic remission is uncertain. The sulphasalazine class of 5-ASA agents failed to demonstrate superiority against placebo, 5-ASAs failed to demonstrate superiority compared to no treatment (very low and low certainty). The efficacy of two different doses of the same 5-ASA and the efficacy of 5-ASA compared to purine antimetabolites (azathioprine or 6-mercaptopurine) in maintaining surgically-induced remission of CD remains unclear. However, purine analogues lead to more serious adverse events and discontinuation due to adverse events. There is a low certainty that 5-ASA is inferior for maintaining surgically-induced remission of CD compared to biologics (anti TNF-ɑ). 5-ASA formulations appear to be safe with no difference in the occurrence of adverse events or withdrawal when compared with placebo, no treatment or biologics.
Conflict of interest statement
Teuta Gjuladin‐Hellon: None known.
Morris Gordon has received travel fees to attend international scientific and training meeting such as DDW, Advances in IBD, ESPGHAN, BSPGHAN and Cochrane focused international events from companies including: Abbott, Nutricia, Biogaia, Ferring, Allergan, and Tillots.
Zipporah Iheozor‐Ejiofor: None known.
Anthony K Akobeng: None known.
Figures
Update of
-
Oral 5-aminosalicylic acid for maintenance of surgically-induced remission in Crohn's disease.Cochrane Database Syst Rev. 2011 Jan 19;(1):CD008414. doi: 10.1002/14651858.CD008414.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2019 Jun 20;6:CD008414. doi: 10.1002/14651858.CD008414.pub3 PMID: 21249709 Updated. Review.
Similar articles
-
Enteral nutrition for maintenance of remission in Crohn's disease.Cochrane Database Syst Rev. 2018 Aug 11;8(8):CD005984. doi: 10.1002/14651858.CD005984.pub3. Cochrane Database Syst Rev. 2018. PMID: 30098021 Free PMC article.
-
Interventions for maintenance of surgically induced remission in Crohn's disease: a network meta-analysis.Cochrane Database Syst Rev. 2019 Sep 12;9(9):CD013210. doi: 10.1002/14651858.CD013210.pub2. Cochrane Database Syst Rev. 2019. PMID: 31513295 Free PMC article.
-
Adalimumab for maintenance of remission in Crohn's disease.Cochrane Database Syst Rev. 2020 May 16;5(5):CD012877. doi: 10.1002/14651858.CD012877.pub2. Cochrane Database Syst Rev. 2020. PMID: 32413933 Free PMC article.
-
Oral 5-aminosalicylic acid for maintenance of surgically-induced remission in Crohn's disease.Cochrane Database Syst Rev. 2011 Jan 19;(1):CD008414. doi: 10.1002/14651858.CD008414.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2019 Jun 20;6:CD008414. doi: 10.1002/14651858.CD008414.pub3 PMID: 21249709 Updated. Review.
-
Aminosalicylates for induction of remission or response in Crohn's disease.Cochrane Database Syst Rev. 2016 Jul 3;7(7):CD008870. doi: 10.1002/14651858.CD008870.pub2. Cochrane Database Syst Rev. 2016. PMID: 27372735 Free PMC article. Review.
Cited by
-
Azathioprine and 6-mercaptopurine for maintenance of surgically-induced remission in Crohn's disease.Cochrane Database Syst Rev. 2019 Aug 6;8(8):CD010233. doi: 10.1002/14651858.CD010233.pub3. Cochrane Database Syst Rev. 2019. PMID: 31425621 Free PMC article.
-
Vitamin D for the treatment of inflammatory bowel disease.Cochrane Database Syst Rev. 2023 Oct 2;10(10):CD011806. doi: 10.1002/14651858.CD011806.pub2. Cochrane Database Syst Rev. 2023. PMID: 37781953 Free PMC article. Review.
-
RNA methylation in inflammatory bowel disease.Cancer Sci. 2024 Mar;115(3):723-733. doi: 10.1111/cas.16048. Epub 2024 Jan 23. Cancer Sci. 2024. PMID: 38263895 Free PMC article. Review.
-
Infliximab for maintenance of medically-induced remission in Crohn's disease.Cochrane Database Syst Rev. 2024 Feb 19;2(2):CD012609. doi: 10.1002/14651858.CD012609.pub2. Cochrane Database Syst Rev. 2024. PMID: 38372447 Review.
-
Maintaining remission in Crohn's disease post surgery: what can we learn from Cochrane?Frontline Gastroenterol. 2024 Jan 17;15(3):241-246. doi: 10.1136/flgastro-2023-102559. eCollection 2024 May. Frontline Gastroenterol. 2024. PMID: 38665790 Review.
References
References to studies included in this review
Ardizzone 2004 {published data only}
-
- Ardizzone S, Maconi G, Sampietro G, Russo A, Radice E, Colombo E, et al. Azathioprine and mesalamine for prevention of relapse after conservative surgery for Crohn's disease. Gastroenterology 2004;127(3):730‐40. - PubMed
Brignola 1995 {published data only}
-
- Brignola C, Cottone M, Pera A, Ardizzone S, Scribano M, Francis R, et al. Mesalamine in the prevention of endoscopic recurrence after intestinal resection for Crohn's disease. Italian Cooperative Study Group. Gastroenterology 1995;108(2):345‐9. - PubMed
Caprilli 1994 {published data only}
-
- Caprilli R, Andreoli A, Capurso L, Corrao G, D'albasio G, Gioieni A, , et al. Oral mesalazine (5‐aminosalicylic acid; Asacol) for the prevention of post‐operative recurrence of Crohn's disease. Gruppo Italiano per lo Studio del Colon e del Retto (GISC). Alimentary Pharmacology & Therapeutics 1994;8(1):35‐43. - PubMed
-
- Caprilli R, Corrao G, Taddei G, Tonelli F, Torchio P, Viscido A. Prognostic factors for postoperative recurrence of Crohn's disease. Diseases of the Colon & Rectum 1996;39(3):335‐41. - PubMed
Caprilli 2003 {published data only}
-
- Caprilli R, Cottone M, Tonelli F, Sturniolo G, Castiglione F, Annese V, et al. Two mesalazine regimens in the prevention of the post‐operative recurrence of Crohns disease: a pragmatic, double‐blind randomized controlled trial. Alimentary Pharmacology & Therapeutics 2003;17(4):517‐23. - PubMed
Ewe 1989 {published data only}
-
- Ewe K, Herfarth C, Malchow H, Jesdinsky HJ. Postoperative recurrence of Crohn's disease in relation to radicality of operation and sulfasalazine prophylaxis: a multicentre trial. Digestion 1989;42(2):224‐32. - PubMed
-
- Ewe K, Holtermuller KH, Baas U, Eckardt V, Kreig H, Kutzner J, et al. Prophylaxis after resection because of Crohn's disease by Salazosulfapyridin (Azulfidine). A double‐blind study [Rezidivprophylaxe nach Darmresektion wegen Morbus Crohn durch Salazosulfapyridin (Azulfidine). Eine Doppelblindstudie]. Verhandlungen der Deutschen Gesellschaft für Innere Medizin 1977;82:930‐2. - PubMed
-
- Ewe K, Malchow H, Herfarth C. Radical operation and recurrence prevention with azulfidine in Crohn disease: a prospective multicenter study‐‐initial results. Langenbeck's Archives of Surgery 1984;364:427‐30. - PubMed
Florent 1996 {published data only}
-
- Florent C, Cortot A, Quandale P, Sahmound T, Modigliani R, Sarfaty E, et al. Placebo‐controlled clinical trial of mesalazine in the prevention of early endoscopic recurrences after resection for Crohn's disease. Groupe d'Etudes Thérapeutiques des Affections Inflammatoires Digestives (GETAID). European Journal of Gastroenterology & Hepatology 1996;8(3):229‐33. - PubMed
Hanauer 2004 {published data only}
-
- Hanauer S, Korelitz B, Rutgeerts P, Peppercorn M, Thisted R, Cohen R, et al. Postoperative maintenance of Crohn's disease remission with 6‐mercaptopurine, mesalamine, or placebo: a 2‐year trial. Gastroenterology 2004;127(3):723‐9. - PubMed
-
- Korelitz BI, Hanauer C, Stephen B, Rutgeerts, Present P, Daniel H, et al. Postoperative prophylaxis with 6‐MP, 5‐ASA or placebo in Crohn's disease: a 2 year multicenter trial. Gastroenterology 1998;114:A1011.
Herfarth 2006 {published data only}
-
- Herfarth H, Obermeier F, Tjaden C, Lukas M, Serclova Z, Dignass AU, et al. Double‐blind, double dummy, randomized, multicentre, comparative study on the efficacy and safety of azathioprine (AZA) versus mesalazine (5‐ASA) for prevention of postoperative endoscopic recurrence in Crohn’s disease. Gastroenterology 2006;130(4 Suppl 2):A480‐1.
-
- Muller R, Herfarth H. More Information on 2006 study published in Gut. Email to Morris Gordon 2/5/2012.
Lochs 2000 {published data only}
-
- Lochs H, Mayer M, Fleig W, Mortensen PB, Bauer P, Genser D, et al. Prophylaxis of postoperative relapse in Crohn's disease with mesalamine: European Cooperative Crohn's Disease Study VI. Gastroenterology 2000;118(2):264‐73. - PubMed
McLeod 1995 {published data only}
-
- McLeod RS, Wolff BG, Steinhart AH, Carryer PW, O'Rourke K, Andrews DF, et al. Prophylactic mesalamine treatment decreases postoperative recurrence of Crohn's disease. Gastroenterology 1995;109(2):404‐13. - PubMed
Reinisch 2010 {published data only}
-
- NCT00946946. Preventing postoperative relapse in Crohn's disease patients at risk: Azathioprine versus mesalazine. clinicaltrials.gov/ct2/show/NCT00946946 (accessed 27 July 2009).
-
- Reinisch W, Angelberger S, Petritsch W, Shonova O, Lukas M, Bar‐Meir S, et al. Azathioprine versus mesalazine for prevention of postoperative clinical recurrence in patients with Crohn's disease with endoscopic recurrence: efficacy and safety results of a randomised, double‐blind, double‐dummy, multicentre trial. Gut 2010;59(6):752‐9. - PubMed
-
- Reinisch W, Angelberger S, Petritsch W, Shonova O, Lukas M, Greinwald R, et al. Azathioprine versus mesalazine for prevention of postoperative clinical recurrence in Crohn's disease patients with endoscopic recurrence: Follow‐up data of a randomised, double‐blind, double‐dummy, 1‐year, multicentre trial. Journal of Crohn's and Colitis 2013;7(Suppl 1):S254. - PubMed
Savarino 2013 {published data only}
-
- Bodini G, Pellegatta G, Giannini EG, Savarino V, Savarino EV. Adalimumab therapy rather than azathioprine and mesalamine is able to halt Crohn's disease progression after resective surgery and a post‐hoc analysis of a prospective randomized study. Gastroenterology 2017;152(5):S774.
-
- Savarino E, Bodini G, Dulbecco P, Assandri L, Bruzzone L, Mazza F. Adalimumab is more effective than azathioprine and mesalamine at preventing postoperative recurrence of Crohn's disease: a randomized controlled trial. American Journal of Gastroenterology 2013;108:1731‐42. - PubMed
Sutherland 1997 {published data only}
-
- Sutherland LR, Martin F, Bailey RJ, Fedorak RN, Poleski M, Dallaire C, et al. A randomized, placebo‐controlled, double‐blind trial of mesalamine in the maintenance of remission of Crohn's disease. The Canadian Mesalamine for Remission of Crohn's Disease Study Group. Gastroenterology 1997;112(4):1069‐77. - PubMed
Wenckert 1978 {published data only}
-
- Wenckert A, Kristensen M, Eklund AE, Barany F, Jarnum S, Worning H, et al. The long‐term prophylactic effect of salazosulphapyridine (salazopyrin) in primarily resected patients with Crohn's disease. A controlled double‐blind trial. Scandinavian Journal of Gastroenterology 1978;13(2):161‐7. - PubMed
References to studies excluded from this review
Dumois 2001 {published data only}
-
- Dumois RA, Herrera JL. Can postoperative relapse of Crohn's disease be prevented?. American Journal of Gastroenterology 2001;96:249. - PubMed
Ewe 1981 {published data only}
-
- Ewe K. Effectiveness of Azulfidine/Salazopyrin in the postoperative prevention of recurrence in Crohn disease. Zeitschrift fur Gastroenterologie 1981;19:41‐4. - PubMed
ISRCTN84003996 {published data only}
-
- ISRCTN84003996. Efficacy of mesalazine to prevent relapse in paediatric Crohn’s disease. isrctn.com/ISRCTN84003996 (accessed 10 March 2008).
McLeod 1997 {published data only}
-
- McLeod RS, Wolff BG, Steinhart AH, Carryer PW, O'Rourke K, Andrews DB, et al. Risk and significance of endoscopic/radiological evidence of recurrent Crohn's disease. Gastroenterology 1997;113(6):1823‐7. - PubMed
NCT00225810 {published data only}
-
- NCT00225810. A study comparing the acceptability of Pentasa® sachets versus Pentasa® tablets in children with Crohn's disease. clinicaltrials.gov/ct2/show/NCT00225810 (accessed 26 September 2005).
NCT00245505 {published data only}
-
- NCT00245505. The effect on mucosal healing with Pentasa sachet in mild to moderate active "Drug: Crohn's Disease". clinicaltrials.gov/ct2/show/NCT00245505 (accessed 28 October 2005).
NCT00300118 {published data only}
-
- NCT00300118. Oral Budesonide vs. oral Mesalazine in active Crohn's disease (CD). clinicaltrials.gov/ct2/show/NCT00300118 (accessed 8 March 2006).
NCT01696942 {published data only}
-
- NCT01696942. Cimzia versus Mesalamine for Crohn's recurrence. clinicaltrials.gov/ct2/show/NCT01696942 (accessed 2 October 2012).
Orlando 2012 {published data only}
-
- Orlando A, Mocciaro F, Scimeca D, Rispo A, Scribano ML, Testa A, et al. Early post‐operative endoscopic recurrence in Crohn's disease patients: Data from a large prospective Italian multicenter cohort. Gastroenterology 2012;1:S259. - PubMed
References to studies awaiting assessment
NCT00976690 {published data only}
-
- NCT00976690. Comparison azathioprine to mesalazine for the prevention of postoperative recurrence in the Crohn disease (IMURELPOST). clinicaltrials.gov/ct2/show/NCT00976690 (accessed 14 September 2009).
Additional references
Abinusawa 2015
Akobeng 2005
Akobeng 2016
Azad Khan 1977
-
- Azad Khan AK, Piris J, Truelove SC. An experiment to determine the active therapeutic moiety of sulphasalazine. Lancet 1977;2(8044):892‐5. - PubMed
Bernell 2000
Boreinstein 2009
-
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta‐analysis. Chichester, West Sussex, UK: John Wiley & Sons Ltd, 2009.
Camma 1997
-
- Camma C, Giunta M, Rosselli M, Cottone M. Mesalamine in the maintenance treatment of Crohns disease: A meta‐analysis adjusted for confounding variables. Gastroenterology 1997;113(5):1465‐73. - PubMed
Das 1973
-
- Das KM, Eastwood MA, McManus JP, Sircus W. Adverse reactions during salicylazosulfapyridine therapy and the relation with drug metabolism and acetylator phenotype. New England Journal of Medicine 1973;289(10):491–5. - PubMed
De Franchis 1997
-
- Franchis R, Omodei P, Ranzi T, Brignola C, Rocca R, Prada A, et al. Controlled trial of oral 5‐aminosalicylic acid for the prevention of early relapse in Crohn's disease. Alimentary Pharmacology & Therapeutics 1997;11(5):845‐52. - PubMed
Gendre 1993
-
- Gendre JP, Mary JY, Florent C, Modigliani R, Colombel JF, Soule JC, et al. Oral mesalamine (Pentasa) as maintenance treatment in Crohn's disease: a multicenter placebo‐controlled study. The Groupe d'Etudes Therapeutiques des Inflammatoires Digestives (GETAID). Gastroenterology 1993;104(2):435‐9. - PubMed
Gklavas 2017
Gordon 2011
Guyatt 2008
Higgins 2011
-
- Higgins JPT, Green S (Editors). Cochrane Handbook for Systematic Reviews of Interventions [Version 5.1.0]. The Cochrane Collaboration, 2011.
Klotz 1980
-
- Klotz U, Maier K, Fischer C, Heinkel KT. Therapeutic efficacy of sulfasalazine and its metabolites in patients with ulcerative colitis and Crohn's disease. New England Journal of Medicine 1980;303(26):1499‐502. - PubMed
Ligumsky 1981
-
- Ligumsky M, Karmeli F, Sharon P, Zor U, Cohen F, Rachmilewitz D. Enhanced thromboxane A2 and prostacyclin production by cultured rectal mucosa in ulcerative colitis and its inhibition by steroids and sulfasalazine. Gastroenterology 1981;81:444–9. - PubMed
Ma 2017
Mahmud 2001
Messori 1994
-
- Messori A, Brignola C, Trallori G, Rampazzo R, Bardazzi G, Belloli C, et al. Effectiveness of 5‐aminosalicylic acid for maintaining remission in patients with Crohn's disease: a meta‐analysis. American Journal of Gastroenterology 1994;89(5):692‐8. - PubMed
Myers 1987
Nguyen 2017
-
- Nguyen GC, Loftus EV Jr, Hirano I, Falck‐Ytter Y, Singh S, Sultan S, et al. American gastroenterological association institute guideline on the management of Crohn’s disease after surgical resection. Gastroenterology 2017;152(1):271‐5. - PubMed
NICE 2012
-
- National Institute for Health and Care Excellence. Crohn's disease: management (clinical guideline). nice.org.uk/guidance/cg152 (accessed 1 October 2012).
NICE 2019
-
- National Institute for Health and Care Excellence. Crohn's disease: management (NICE guideline NG129). nice.org.uk/guidance/ng129 (accessed 3 May 2019).
Nugent 2001
Rasmussen 1982
-
- Rasmussen SN, Bondesen S, Hvidberg EF. 5‐Aminosalicylic acid in a slow‐release preparation. Advances in Therapy 2015;32:477‐84. - PubMed
Rousseaux 2005
Rutgeerts 1990
-
- Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohns disease. Gastroenterology 1990;99(4):956‐63. - PubMed
Rutgeerts 2002
Schroder 1972
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. In: Higgins JPT, Green S, editor(s). Chapter 12: Interpreting results and drawing conclusions. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Shafil 1982
-
- Shafii A, Chowdhury JR, Das KM. Absorption, enterohepatic circulation, and excretion of 5‐aminosalicylic acid in rats. American Journal of Gastroenterology 1982;77:297–9. - PubMed
Steinhart 1994
-
- Steinhart AH, Hemphill D, Greenberg GR. Sulfasalazine and mesalazine for the maintenance therapy of Crohn's disease: a meta‐analysis. American Journal of Gastroenterology 1994;89(12):2116‐24. - PubMed
Sutton 2000
-
- Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for meta‐analysis in medical research. Chichester, West Sussex, UK: John Wiley & Sons Ltd, 2000.
Svartz 1942
-
- Svartz N. Salazopyrin A. Salazopyrin, a new sulfanilamide preparation. Acta Medica Scandinavica 1942;110:557‐90.
Van Assche 2010
-
- Assche G, Dignass A, Reinisch W. The second European evidence‐based consensus on the diagnosis and management of Crohn's disease: Special situations. Journal of Crohn's and Colitis 2010;4:63‐101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical