Interferon-γ induces the cell surface exposure of phosphatidylserine by activating the protein MLKL in the absence of caspase-8 activity
- PMID: 31217278
- PMCID: PMC6690710
- DOI: 10.1074/jbc.RA118.007161
Interferon-γ induces the cell surface exposure of phosphatidylserine by activating the protein MLKL in the absence of caspase-8 activity
Abstract
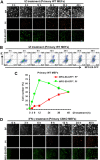
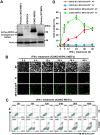
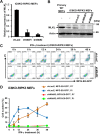
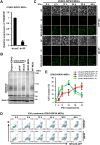
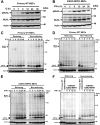
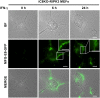
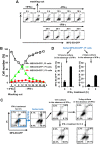
Phosphatidylserine (PS), an anionic phospholipid enriched in the inner leaflet of the plasma membrane, is exposed to the outer leaflet during apoptosis. PS exposure was recently shown to be induced during tumor necrosis factor-induced necroptosis. We herein demonstrated that interferon (IFN)-γ induced necroptosis in Caspase-8-knockout mouse-derived embryonic fibroblasts (C8KO MEFs), as well as in WT MEFs co-treated with the pan-caspase inhibitor, z-VAD-fmk. PS exposure and necroptosis were significant after 6- and 24-h treatments with IFN-γ, respectively. To elucidate the molecular mechanisms underlying IFN-γ-induced PS exposure, we generated C8KO MEF-derived cell lines without the expression of RIPK3 (receptor-interacting protein kinase 3), an essential molecule in tumor necrosis factor-induced necroptosis, and IFN-γ-induced PS exposure and necrotic cell death were shown to be specifically inhibited by the loss of RIPK3 expression. Furthermore, the down-regulated expression of MLKL (mixed lineage kinase domain-like protein), a key molecule for inducing membrane rupture downstream of RIPK3 in necroptosis, abolished IFN-γ-induced PS exposure in C8KO MEFs. In human colorectal adenocarcinoma-derived HT29 cells, PS exposure and necroptosis were similarly induced by treatment with IFN-γ in the presence of Smac mimetics and z-VAD-fmk. The removal of IFN-γ from PS-exposing MEFs after a 6-h treatment completely inhibited necroptotic cell death but not the subsequent increase in the number of PS-exposing cells. Therefore, PS exposure mediated by RIPK3-activated MLKL oligomers was induced by a treatment with IFN-γ for a significant interval of time before the induction of necroptosis by membrane rupture.
Keywords: MLKL; RIPK3; caspase; cell death; interferon; interferon-gamma; necroptosis; necrosis (necrotic death); phosphatidylserine; receptor-interacting protein (RIP).
© 2019 Chen et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
Similar articles
-
The neurotoxicant PCB-95 by increasing the neuronal transcriptional repressor REST down-regulates caspase-8 and increases Ripk1, Ripk3 and MLKL expression determining necroptotic neuronal death.Biochem Pharmacol. 2017 Oct 15;142:229-241. doi: 10.1016/j.bcp.2017.06.135. Epub 2017 Jul 1. Biochem Pharmacol. 2017. PMID: 28676433
-
Necroptosis induced by RIPK3 requires MLKL but not Drp1.Cell Death Dis. 2014 Feb 27;5(2):e1086. doi: 10.1038/cddis.2014.18. Cell Death Dis. 2014. PMID: 24577084 Free PMC article.
-
Key necroptotic proteins are required for Smac mimetic-mediated sensitization of cholangiocarcinoma cells to TNF-α and chemotherapeutic gemcitabine-induced necroptosis.PLoS One. 2020 Jan 8;15(1):e0227454. doi: 10.1371/journal.pone.0227454. eCollection 2020. PLoS One. 2020. PMID: 31914150 Free PMC article.
-
Necroptosis in development, inflammation and disease.Nat Rev Mol Cell Biol. 2017 Feb;18(2):127-136. doi: 10.1038/nrm.2016.149. Epub 2016 Dec 21. Nat Rev Mol Cell Biol. 2017. PMID: 27999438 Review.
-
The Inflammatory Signal Adaptor RIPK3: Functions Beyond Necroptosis.Int Rev Cell Mol Biol. 2017;328:253-275. doi: 10.1016/bs.ircmb.2016.08.007. Epub 2016 Sep 22. Int Rev Cell Mol Biol. 2017. PMID: 28069136 Free PMC article. Review.
Cited by
-
In situ vaccination caused by diverse irradiation-driven cell death programs.Theranostics. 2024 Jan 12;14(3):1147-1167. doi: 10.7150/thno.86004. eCollection 2024. Theranostics. 2024. PMID: 38323315 Free PMC article. Review.
-
Interferon-mediated repression of miR-324-5p potentiates necroptosis to facilitate antiviral defense.EMBO Rep. 2022 Aug 3;23(8):e54438. doi: 10.15252/embr.202154438. Epub 2022 Jun 23. EMBO Rep. 2022. PMID: 35735238 Free PMC article.
-
Induction of Immune Responses and Phosphatidylserine Exposure by TLR9 Activation Results in a Cooperative Antitumor Effect with a Phosphatidylserine-targeting Prodrug.Int J Biol Sci. 2023 May 11;19(9):2648-2662. doi: 10.7150/ijbs.81683. eCollection 2023. Int J Biol Sci. 2023. PMID: 37324949 Free PMC article.
-
Update of cellular responses to the efferocytosis of necroptosis and pyroptosis.Cell Div. 2023 Apr 9;18(1):5. doi: 10.1186/s13008-023-00087-6. Cell Div. 2023. PMID: 37032375 Free PMC article. Review.
-
Flipping the dogma - phosphatidylserine in non-apoptotic cell death.Cell Commun Signal. 2019 Oct 29;17(1):139. doi: 10.1186/s12964-019-0437-0. Cell Commun Signal. 2019. PMID: 31665027 Free PMC article. Review.
References
-
- Fadok V. A., Voelker D. R., Campbell P. A., Cohen J. J., Bratton D. L., and Henson P. M. (1992) Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148, 2207–2216 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

