Hematopoietic progenitor cells as integrative hubs for adaptation to and fine-tuning of inflammation
- PMID: 31213716
- PMCID: PMC6709414
- DOI: 10.1038/s41590-019-0402-5
Hematopoietic progenitor cells as integrative hubs for adaptation to and fine-tuning of inflammation
Abstract
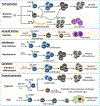
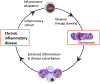
Recent advances have highlighted the ability of hematopoietic stem and progenitor cells in the bone marrow to sense peripheral inflammation or infection and adapt through increased proliferation and skewing toward the myeloid lineage. Such adaptations can meet the increased demand for innate immune cells and can be beneficial in response to infection or myeloablation. However, the inflammation-induced adaptation of hematopoietic and myeloid progenitor cells toward enhanced myelopoiesis might also perpetuate inflammation in chronic inflammatory or cardio-metabolic diseases by generating a feed-forward loop between inflammation-adapted hematopoietic progenitor cells and the inflammatory disorder. Sustained adaptive responses of progenitor cells in the bone marrow can also contribute to trained immunity, a non-specific memory of earlier encounters that in turn facilitates the heightened response of these cells, as well as that of their progeny, to future challenges. Here we discuss the mechanisms that govern the adaptation of hematopoietic progenitor cells to inflammation and its sequelae in the pathogenesis of human disease.
Figures
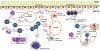

Similar articles
-
Hematopoietic Progenitors and the Bone Marrow Niche Shape the Inflammatory Response and Contribute to Chronic Disease.Int J Mol Sci. 2022 Feb 17;23(4):2234. doi: 10.3390/ijms23042234. Int J Mol Sci. 2022. PMID: 35216355 Free PMC article. Review.
-
Myelopoiesis in the Context of Innate Immunity.J Innate Immun. 2018;10(5-6):365-372. doi: 10.1159/000489406. Epub 2018 Jun 6. J Innate Immun. 2018. PMID: 29874678 Free PMC article. Review.
-
Regulation of the Bone Marrow Niche by Inflammation.Front Immunol. 2020 Jul 21;11:1540. doi: 10.3389/fimmu.2020.01540. eCollection 2020. Front Immunol. 2020. PMID: 32849521 Free PMC article. Review.
-
Trained Immunity and Cardiometabolic Disease: The Role of Bone Marrow.Arterioscler Thromb Vasc Biol. 2021 Jan;41(1):48-54. doi: 10.1161/ATVBAHA.120.314215. Epub 2020 Nov 19. Arterioscler Thromb Vasc Biol. 2021. PMID: 33207931 Free PMC article. Review.
-
Promotion of Expansion and Differentiation of Hematopoietic Stem Cells by Interleukin-27 into Myeloid Progenitors to Control Infection in Emergency Myelopoiesis.PLoS Pathog. 2016 Mar 18;12(3):e1005507. doi: 10.1371/journal.ppat.1005507. eCollection 2016 Mar. PLoS Pathog. 2016. PMID: 26991425 Free PMC article.
Cited by
-
Sexually dimorphic role of diet and stress on behavior, energy metabolism, and the ventromedial hypothalamus.bioRxiv [Preprint]. 2023 Nov 17:2023.11.17.567534. doi: 10.1101/2023.11.17.567534. bioRxiv. 2023. Update in: Biol Sex Differ. 2024 Jul 15;15(1):55. doi: 10.1186/s13293-024-00628-w PMID: 38014350 Free PMC article. Updated. Preprint.
-
TICAM2-related pathway mediates neutrophil exhaustion.Sci Rep. 2020 Sep 1;10(1):14397. doi: 10.1038/s41598-020-71379-y. Sci Rep. 2020. PMID: 32873853 Free PMC article.
-
Neutrophil phenotypes and functions in cancer: A consensus statement.J Exp Med. 2022 Jun 6;219(6):e20220011. doi: 10.1084/jem.20220011. Epub 2022 May 6. J Exp Med. 2022. PMID: 35522219 Free PMC article. Review.
-
Myelodysplastic Syndromes and Metabolism.Int J Mol Sci. 2021 Oct 19;22(20):11250. doi: 10.3390/ijms222011250. Int J Mol Sci. 2021. PMID: 34681910 Free PMC article. Review.
-
Association between Periodontal Disease and Arteriosclerosis-Related Diseases.J Atheroscler Thromb. 2023 Nov 1;30(11):1517-1524. doi: 10.5551/jat.RV22010. Epub 2023 Aug 29. J Atheroscler Thromb. 2023. PMID: 37648470 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

