Aronia Berry Supplementation Mitigates Inflammation in T Cell Transfer-Induced Colitis by Decreasing Oxidative Stress
- PMID: 31212794
- PMCID: PMC6627224
- DOI: 10.3390/nu11061316
Aronia Berry Supplementation Mitigates Inflammation in T Cell Transfer-Induced Colitis by Decreasing Oxidative Stress
Abstract
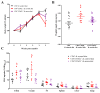
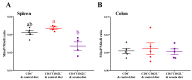
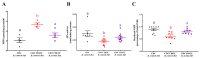
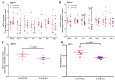
Oxidative stress is involved in the pathogenesis and progression of inflammatory bowel disease. Consumption of aronia berry inhibits T cell transfer colitis, but the antioxidant mechanisms pertinent to immune function are unclear. We hypothesized that aronia berry consumption could inhibit inflammation by modulating the antioxidant function of immunocytes and gastrointestinal tissues. Colitis was induced in recombinase activating gene-1 deficient (Rag1-/-) mice injected with syngeneic CD4+CD62L+ naïve T cells. Concurrent with transfer, mice consumed either 4.5% w/w aronia berry-supplemented or a control diet for five weeks. Aronia berry inhibited intestinal inflammation evidenced by lower colon weight/length ratios, 2-deoxy-2-[18F]fluoro-d-glucose (FDG) uptake, mRNA expressions of tumor necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ) in the colon. Aronia berry also suppressed systemic inflammation evidenced by lower FDG uptake in the spleen, liver, and lung. Colitis induced increased colon malondialdehyde (MDA), decreased colon glutathione peroxidase (GPx) activity, reduced glutathione (rGSH) level, and suppressed expression of antioxidant enzymes in the colon and mesenteric lymph node (MLN). Aronia berry upregulated expression of antioxidant enzymes, prevented colitis-associated depletion of rGSH, and maintained GPx activity. Moreover, aronia berry modulated mitochondria-specific antioxidant activity and decreased splenic mitochondrial H2O2 production in colitic mice. Thus, aronia berry consumption inhibits oxidative stress in the colon during T cell transfer colitis because of its multifaceted antioxidant function in both the cytosol and mitochondria of immunocytes.
Keywords: adoptive transfer colitis; aronia berry; inflammatory bowel disease; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Dietary Prevention of Colitis by Aronia Berry is Mediated Through Increased Th17 and Treg.Mol Nutr Food Res. 2019 Mar;63(5):e1800985. doi: 10.1002/mnfr.201800985. Epub 2018 Dec 13. Mol Nutr Food Res. 2019. PMID: 30521111
-
Aronia Berry Extract Ameliorates the Severity of Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice.J Med Food. 2017 Jul;20(7):667-675. doi: 10.1089/jmf.2016.3822. Epub 2017 Jul 5. J Med Food. 2017. PMID: 28677983
-
The Effect of Aronia Berry on Type 1 Diabetes In Vivo and In Vitro.J Med Food. 2018 Mar;21(3):244-253. doi: 10.1089/jmf.2017.3939. Epub 2018 Feb 22. J Med Food. 2018. PMID: 29470134
-
Daily supplementation with aronia melanocarpa (chokeberry) reduces blood pressure and cholesterol: a meta analysis of controlled clinical trials.J Diet Suppl. 2021;18(5):517-530. doi: 10.1080/19390211.2020.1800887. Epub 2020 Aug 14. J Diet Suppl. 2021. PMID: 32794414 Review.
-
Berry Phenolic Antioxidants - Implications for Human Health?Front Pharmacol. 2018 Mar 26;9:78. doi: 10.3389/fphar.2018.00078. eCollection 2018. Front Pharmacol. 2018. PMID: 29662448 Free PMC article. Review.
Cited by
-
Chokeberry (A. melanocarpa (Michx.) Elliott)-A Natural Product for Metabolic Disorders?Nutrients. 2022 Jun 28;14(13):2688. doi: 10.3390/nu14132688. Nutrients. 2022. PMID: 35807867 Free PMC article. Review.
-
Effects of Chokeberries (Aronia spp.) on Cytoprotective and Cardiometabolic Markers and Semen Quality in 109 Mildly Hypercholesterolemic Danish Men: A Prospective, Double-Blinded, Randomized, Crossover Trial.J Clin Med. 2023 Jan 3;12(1):373. doi: 10.3390/jcm12010373. J Clin Med. 2023. PMID: 36615174 Free PMC article.
-
Dietary Anthocyanins and Human Health.Nutrients. 2019 Sep 5;11(9):2107. doi: 10.3390/nu11092107. Nutrients. 2019. PMID: 31491856 Free PMC article.
-
Cellular Antioxidant Effect of an Aronia Extract and Its Polyphenolic Fractions Enriched in Proanthocyanidins, Phenolic Acids, and Anthocyanins.Antioxidants (Basel). 2022 Aug 12;11(8):1561. doi: 10.3390/antiox11081561. Antioxidants (Basel). 2022. PMID: 36009281 Free PMC article.
-
Consumption of Chokeberry Bio-Products Improves Specific Metabolic Parameters and Increases the Plasma Antioxidant Status.Antioxidants (Basel). 2024 Jun 7;13(6):699. doi: 10.3390/antiox13060699. Antioxidants (Basel). 2024. PMID: 38929138 Free PMC article.
References
-
- Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol E.I., Panaccione R., Ghosh S., Wu J.C.Y., Chan F.K.L., et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

