Antibody-drug conjugates targeting TROP-2 and incorporating SN-38: A case study of anti-TROP-2 sacituzumab govitecan
- PMID: 31208270
- PMCID: PMC6748572
- DOI: 10.1080/19420862.2019.1632115
Antibody-drug conjugates targeting TROP-2 and incorporating SN-38: A case study of anti-TROP-2 sacituzumab govitecan
Abstract
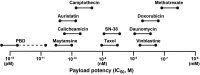
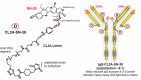
Antibody-drug conjugates (ADCs) that exploit the active metabolite SN-38, which is derived from the popular anticancer drug, irinotecan (a camptothecin that inhibits the nuclear topoisomerase I enzyme, inducing double-stranded DNA breaks during the mitotic S-phase of affected cells), represent a substantial advance in the ADC field. SN-38 has been conjugated to a humanized antibody against trophoblast cell surface antigen 2 (TROP-2), which is involved in cancer signaling pathways and has increased expression by many cancer cell types, yielding the ADC sacituzumab govitecan. By conjugating a higher number of SN-38 molecules to the immunoglobulin (drug-to-antibody ratio = 7-8:1), and giving higher (10 mg/kg) and repeated therapy cycles (Days 1 and 8 of 21-day cycles), enhanced drug uptake by the targeted cancer cells is achieved. Based on a unique conjugation method, the lactone ring of the SN-38 molecule is stabilized and the molecule is protected from glucuronidation, a process that contributes to the untoward late diarrhea experienced with irinotecan. Finally, while the ADC is internalized, the use of a moderately stable linker permits release of SN-38 in an acidic environment of the tumor cell and its microenvironment, contributing to a bystander effect on neighboring cancer cells. Here, we discuss the development of sacituzumab govitecan and clinical results obtained using it for the management of patients with advanced, refractive breast, lung, and urinary bladder cancers. Sacituzumab govitecan, which is undergoing accelerated approval review by the US Food and Drug Administration while also being studied in Phase 3 clinical studies, was granted Breakthrough Therapy status from the FDA for advanced, refractory, metastatic triple-negative breast cancer patients.
Keywords: Antibody-drug conjugate; IMMU-132; SN-38; TROP-2; sacituzumab govitecan.
Figures
Similar articles
-
Sacituzumab Govitecan (IMMU-132), an Anti-Trop-2/SN-38 Antibody-Drug Conjugate: Characterization and Efficacy in Pancreatic, Gastric, and Other Cancers.Bioconjug Chem. 2015 May 20;26(5):919-31. doi: 10.1021/acs.bioconjchem.5b00223. Epub 2015 May 8. Bioconjug Chem. 2015. PMID: 25915780
-
Sacituzumab govitecan, a novel, third-generation, antibody-drug conjugate (ADC) for cancer therapy.Expert Opin Biol Ther. 2020 Aug;20(8):871-885. doi: 10.1080/14712598.2020.1757067. Epub 2020 May 12. Expert Opin Biol Ther. 2020. PMID: 32301634 Review.
-
Sacituzumab govitecan (IMMU-132), an anti-Trop-2-SN-38 antibody-drug conjugate for the treatment of diverse epithelial cancers: Safety and pharmacokinetics.Cancer. 2017 Oct 1;123(19):3843-3854. doi: 10.1002/cncr.30789. Epub 2017 May 30. Cancer. 2017. PMID: 28558150 Clinical Trial.
-
Sacituzumab govitecan: antibody-drug conjugate in triple-negative breast cancer and other solid tumors.Drugs Today (Barc). 2019 Sep;55(9):575-585. doi: 10.1358/dot.2019.55.9.3039669. Drugs Today (Barc). 2019. PMID: 31584574 Free PMC article. Review.
-
First-in-Human Trial of a Novel Anti-Trop-2 Antibody-SN-38 Conjugate, Sacituzumab Govitecan, for the Treatment of Diverse Metastatic Solid Tumors.Clin Cancer Res. 2015 Sep 1;21(17):3870-8. doi: 10.1158/1078-0432.CCR-14-3321. Epub 2015 May 5. Clin Cancer Res. 2015. PMID: 25944802 Free PMC article. Clinical Trial.
Cited by
-
Delivery of Drugs into Cancer Cells Using Antibody-Drug Conjugates Based on Receptor-Mediated Endocytosis and the Enhanced Permeability and Retention Effect.Antibodies (Basel). 2022 Dec 19;11(4):78. doi: 10.3390/antib11040078. Antibodies (Basel). 2022. PMID: 36546903 Free PMC article. Review.
-
Sacituzumab govitecan response in extensive leptomeningeal carcinomatosis from triple-negative breast cancer: a case report.Front Oncol. 2024 Aug 12;14:1378248. doi: 10.3389/fonc.2024.1378248. eCollection 2024. Front Oncol. 2024. PMID: 39188688 Free PMC article.
-
Trophoblast Cell Surface Antigen 2 (TROP2) as a Predictive Bio-Marker for the Therapeutic Efficacy of Sacituzumab Govitecan in Adenocarcinoma of the Esophagus.Cancers (Basel). 2022 Sep 30;14(19):4789. doi: 10.3390/cancers14194789. Cancers (Basel). 2022. PMID: 36230712 Free PMC article.
-
The pathological and clinical landscape of refractory metastatic triple negative breast cancer: a narrative review.Ann Transl Med. 2022 Aug;10(16):907. doi: 10.21037/atm-22-3434. Ann Transl Med. 2022. PMID: 36111045 Free PMC article. Review.
-
Current Analytical Strategies for Antibody-Drug Conjugates in Biomatrices.Molecules. 2022 Sep 24;27(19):6299. doi: 10.3390/molecules27196299. Molecules. 2022. PMID: 36234836 Free PMC article. Review.
References
-
- Abdollahpour-Alitappeh M, Lotfinia M, Gharibi T, Mardaneh J, Farhadihosseinabadi B, Larki P, Faghfourian B, Sepehr KS, Abbaszadeh-Goudarzi K, Abbaszadeh-Goudarzi G, et al. Antibody-drug conjugates (ADCs) for cancer therapy: strategies, challenges, and successes. J Cell Physiol. 2019;234:5628–42. doi:10.1002/jcp.27419. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
