AURIEL-PsO: a randomized, double-blind phase III equivalence trial to demonstrate the clinical similarity of the proposed biosimilar MSB11022 to reference adalimumab in patients with moderate-to-severe chronic plaque-type psoriasis
- PMID: 31206593
- PMCID: PMC7027805
- DOI: 10.1111/bjd.18220
AURIEL-PsO: a randomized, double-blind phase III equivalence trial to demonstrate the clinical similarity of the proposed biosimilar MSB11022 to reference adalimumab in patients with moderate-to-severe chronic plaque-type psoriasis
Abstract
Background: MSB11022 is a proposed adalimumab biosimilar.
Objectives: To compare the efficacy, safety and immunogenicity of MSB11022 with reference adalimumab.
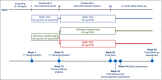
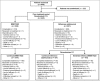
Methods: AURIEL-PsO was a double-blind randomized controlled equivalence trial, in which patients with moderate-to-severe chronic plaque-type psoriasis were randomized 1 : 1 to MSB11022 or reference adalimumab. The primary end point was ≥ 75% improvement in Psoriasis Area and Severity Index (PASI 75) at week 16, with a prespecified equivalence interval of ± 18%. Patients with a ≥50% improvement in PASI at week 16 were eligible to enter a double-blind extension period: patients receiving MSB11022 continued treatment, and patients receiving reference adalimumab were rerandomized 1 : 1 either to continue reference adalimumab or to switch to MSB11022. Other efficacy end points and safety, immunogenicity and pharmacokinetic parameters were evaluated at scheduled visits up to weeks 52 (efficacy and immunogenicity), 54 and 66 (safety).
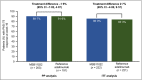
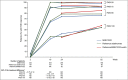
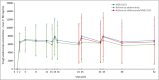
Results: In total, 443 patients were randomized. The difference in PASI 75 response rates at week 16 between the treatment arms was -1·9%, and the 95% confidence interval (-7·8% to 4·1%) was within the prespecified equivalence interval. No notable difference in the incidence of treatment-emergent adverse events was observed between treatment arms up to the end of the trial, and no new safety signals were observed. Following treatment switch at week 16, no clinically meaningful differences in safety or immunogenicity were seen between treatment arms through to the end of the observation period.
Conclusions: Therapeutic equivalence between MSB11022 and reference adalimumab was demonstrated. AURIEL-PsO provides evidence to support the similarity of both products with regard to efficacy, safety and immunogenicity. What's already known about this topic? Adalimumab is a fully human antitumour necrosis factor-α monoclonal antibody, indicated for the treatment of multiple inflammatory disorders, including psoriasis, psoriatic arthritis, rheumatoid arthritis, inflammatory bowel diseases and ankylosing spondylitis. MSB11022 is a proposed adalimumab biosimilar that has shown structural and functional similarity to the reference product in an extensive analytical comparability exercise. MSB11022 has demonstrated bioequivalence and comparable safety and immunogenicity profiles in a phase I study in healthy volunteers. What does this study add? This phase III study confirmed equivalent efficacy for MSB11022 and reference adalimumab in patients without any immunomodulation comedication in moderate-to-severe chronic plaque-type psoriasis at week 16. The efficacy, safety and immunogenicity of MSB11022 and reference adalimumab were similar over the respective observation periods (week 52 for efficacy and immunogenicity, week 66 for safety). A switch from reference adalimumab to MSB11022 at week 16 did not impact efficacy, safety or immunogenicity.
© 2019 The Authors. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Conflict of interest statement
J.H. has received honoraria for attendance at advisory boards for Novartis, Eli Lilly, LEO Pharma, Nordic Pharma, UCB, Sanofi Genzyme and Fresenius Kabi; as an investigator for AbbVie, Merck, Amgen, Novartis, Eli Lilly and Pfizer; and as a speaker for AbbVie, Biogen, Eli Lilly, Janssen‐Cilag, LEO Pharma, L'Oréal, Nordic Pharma, Novartis, Pfizer, Pierre Fabre and Sanofi‐Aventis. K.A.P. has received honoraria for attendance at advisory boards for AbbVie, Amgen, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Dow Pharma, Eli Lilly, Fresenius Kabi, Galderma, Janssen, Merck (MSD), Novartis, Pfizer, Regeneron, Sanofi‐Aventis/Genzyme, UCB and Valeant; as a speaker for AbbVie, Amgen, Celgene, Eli Lilly, Galderma, Janssen, Kyowa Hakka Kirin, LEO, Merck (MSD), Novartis, Pfizer and Valeant; as a consultant for AbbVie, Akros, Amgen, Baxalta, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Coherus, Dermira, Dow Pharma, Eli Lilly, Galderma, Janssen, Kyowa Hakka Kirin, LEO, Merck (MSD), Merck‐Serono, Novartis, Pfizer, Regeneron, Roche, Sanofi‐Aventis/Genzyme, Takeda, UCB and Valeant; and for other activities for AbbVie, Akros, Amgen, Anacor, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, Kyowa Hakka Kirin, Merck (MSD), Merck‐Serono, Novartis, Pfizer, Regeneron, Sanofi‐Aventis/Genzyme and Valeant; and has received grants as an investigator for AbbVie, Akros, Amgen, Anacor, Baxalta, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Coherus, Dermira, Dow Pharma, Eli Lilly, Galderma, GSK, Janssen, Kyowa Hakka Kirin, LEO, Merck (MSD), Merck‐Serono, Novartis, Pfizer, Regeneron, Roche, Sanofi‐Aventis/Genzyme, Takeda, UCB and Valeant. V.C. is a former employee of Fresenius Kabi SwissBioSim. M.U. is an employee of Fresenius Kabi SwissBioSim. P.V. has no conflicts of interest to declare. C.J.E. has received honoraria for attendance at advisory boards for AbbVie, Biogen, BMS, Celgene, Fresenius Kabi, GSK, Janssen, Lilly, Mundipharma, Roche and Sanofi; and as a consultant for Anthera, Merck and Samsung Bioepis; and has received grants as an investigator for AbbVie, Biogen and Pfizer.
Figures

Comment in
-
Noninferiority of biosimilar MSB110222 to reference adalimumab for chronic plaque psoriasis.Br J Dermatol. 2020 Feb;182(2):266. doi: 10.1111/bjd.18717. Epub 2019 Dec 15. Br J Dermatol. 2020. PMID: 31840231 No abstract available.
Similar articles
-
Efficacy and Safety of HLX03, an Adalimumab Biosimilar, in Patients with Moderate-to-Severe Plaque Psoriasis: A Randomized, Double-Blind, Phase III Study.Adv Ther. 2022 Jan;39(1):583-597. doi: 10.1007/s12325-021-01899-0. Epub 2021 Nov 23. Adv Ther. 2022. PMID: 34816373 Free PMC article. Clinical Trial.
-
Safety of adalimumab biosimilar MSB11022 (acetate-buffered formulation) in patients with moderately-to-severely active rheumatoid arthritis.Clin Rheumatol. 2019 Dec;38(12):3381-3390. doi: 10.1007/s10067-019-04679-y. Epub 2019 Aug 8. Clin Rheumatol. 2019. PMID: 31396834 Clinical Trial.
-
Phase III randomized study of the proposed adalimumab biosimilar GP2017 in psoriasis: impact of multiple switches.Br J Dermatol. 2018 Sep;179(3):623-631. doi: 10.1111/bjd.16890. Epub 2018 Jul 15. Br J Dermatol. 2018. PMID: 29917226 Clinical Trial.
-
A Review of the Totality of Evidence Supporting the Development of the First Adalimumab Biosimilar ABP 501.Adv Ther. 2019 Aug;36(8):1833-1850. doi: 10.1007/s12325-019-00979-6. Epub 2019 Jun 10. Adv Ther. 2019. PMID: 31183781 Free PMC article. Review.
-
An Update Review of Biosimilars of Adalimumab in Psoriasis - Bioequivalence and Interchangeability.Drug Des Devel Ther. 2021 Jul 8;15:2987-2998. doi: 10.2147/DDDT.S317382. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 34267501 Free PMC article. Review.
Cited by
-
An Introduction to Biosimilars for the Treatment of Retinal Diseases: A Narrative Review.Ophthalmol Ther. 2022 Jun;11(3):959-982. doi: 10.1007/s40123-022-00488-w. Epub 2022 Mar 12. Ophthalmol Ther. 2022. PMID: 35278204 Free PMC article. Review.
-
Efficacy and Safety of HLX03, an Adalimumab Biosimilar, in Patients with Moderate-to-Severe Plaque Psoriasis: A Randomized, Double-Blind, Phase III Study.Adv Ther. 2022 Jan;39(1):583-597. doi: 10.1007/s12325-021-01899-0. Epub 2021 Nov 23. Adv Ther. 2022. PMID: 34816373 Free PMC article. Clinical Trial.
-
Efficacy and Safety of Adalimumab Biosimilars: Current Critical Clinical Data in Rheumatoid Arthritis.Front Immunol. 2021 Apr 6;12:638444. doi: 10.3389/fimmu.2021.638444. eCollection 2021. Front Immunol. 2021. PMID: 33889152 Free PMC article. Review.
-
Efficacy and Safety of Anti-TNF Biosimilars for Psoriasis in Pediatric and Geriatric Populations: A 72-Week Real-Life Study.Psoriasis (Auckl). 2022 Jul 9;12:199-204. doi: 10.2147/PTT.S365493. eCollection 2022. Psoriasis (Auckl). 2022. PMID: 35844291 Free PMC article.
-
Persistence and safety of anti-TNF biosimilars versus originators in immune-mediated inflammatory diseases: an observational study on the French National Health Data System.RMD Open. 2024 Mar 6;10(1):e003531. doi: 10.1136/rmdopen-2023-003531. RMD Open. 2024. PMID: 38453213 Free PMC article.
References
-
- Feldmann M, Maini RN. Anti‐TNF therapy, from rationale to standard of care: what lessons has it taught us? J Immunol 2010; 185:791–4. - PubMed
-
- Kaymakcalan Z, Sakorafas P, Bose S et al Comparisons of affinities, avidities, and complement activation of adalimumab, infliximab, and etanercept in binding to soluble and membrane tumor necrosis factor. Clin Immunol 2009; 131:308–16. - PubMed
-
- Lapadula G, Marchesoni A, Armuzzi A et al Adalimumab in the treatment of immune‐mediated diseases. Int J Immunopathol Pharmacol 2014; 27:33–48. - PubMed
-
- Putrik P, Ramiro S, Kvien TK et al Variations in criteria regulating treatment with reimbursed biologic DMARDs across European countries. Are differences related to country's wealth? Ann Rheum Dis 2014; 73:2010–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

