Novel Dimethyltyrosine-Tetrahydroisoquinoline Peptidomimetics with Aromatic Tetrahydroisoquinoline Substitutions Show in Vitro Kappa and Mu Opioid Receptor Agonism
- PMID: 31199621
- PMCID: PMC6726392
- DOI: 10.1021/acschemneuro.9b00250
Novel Dimethyltyrosine-Tetrahydroisoquinoline Peptidomimetics with Aromatic Tetrahydroisoquinoline Substitutions Show in Vitro Kappa and Mu Opioid Receptor Agonism
Abstract
The dimethyltyrosine-tetrahydroisoquinoline (Dmt-Tiq) scaffold was originally developed in the production of selective delta opioid receptor (DOR) antagonists. Installation of a 7-benzyl pendant on the tetrahydroisoquinoline core of this classic opioid scaffold introduced kappa opioid receptor (KOR) agonism. Further modification of this pendant resulted in retention of KOR agonism and the addition of mu opioid receptor (MOR) partial agonism, a bifunctional profile with potential to be used in the treatment of cocaine addiction.
Keywords: cocaine addiction; dimethyltyrosine−tetrahydroisoquinoline; multifunctional ligands; opioids; peptidomimetics; synthesis.
Figures
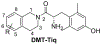

Similar articles
-
Structure-Activity Relationships of 7-Substituted Dimethyltyrosine-Tetrahydroisoquinoline Opioid Peptidomimetics.Molecules. 2019 Nov 26;24(23):4302. doi: 10.3390/molecules24234302. Molecules. 2019. PMID: 31779072 Free PMC article.
-
SAR Matrices Enable Discovery of Mixed Efficacy μ-Opioid Receptor Agonist Peptidomimetics with Simplified Structures through an Aromatic-Amine Pharmacophore.ACS Chem Neurosci. 2021 Jan 6;12(1):216-233. doi: 10.1021/acschemneuro.0c00693. Epub 2020 Dec 21. ACS Chem Neurosci. 2021. PMID: 33346631 Free PMC article.
-
Placement of Hydroxy Moiety on Pendant of Peptidomimetic Scaffold Modulates Mu and Kappa Opioid Receptor Efficacy.ACS Chem Neurosci. 2017 Nov 15;8(11):2549-2557. doi: 10.1021/acschemneuro.7b00284. Epub 2017 Aug 25. ACS Chem Neurosci. 2017. PMID: 28796483 Free PMC article.
-
Potential for Kappa-Opioid Receptor Agonists to Engineer Nonaddictive Analgesics: A Narrative Review.Anesth Analg. 2021 Feb 1;132(2):406-419. doi: 10.1213/ANE.0000000000005309. Anesth Analg. 2021. PMID: 33332902 Free PMC article. Review.
-
The search for opioid analgesics with limited tolerance liability.Peptides. 2020 Aug;130:170331. doi: 10.1016/j.peptides.2020.170331. Epub 2020 Jun 1. Peptides. 2020. PMID: 32497566 Review.
Cited by
-
Peptidomimetics and Their Applications for Opioid Peptide Drug Discovery.Biomolecules. 2022 Sep 5;12(9):1241. doi: 10.3390/biom12091241. Biomolecules. 2022. PMID: 36139079 Free PMC article. Review.
-
Structure-Activity Relationships of 7-Substituted Dimethyltyrosine-Tetrahydroisoquinoline Opioid Peptidomimetics.Molecules. 2019 Nov 26;24(23):4302. doi: 10.3390/molecules24234302. Molecules. 2019. PMID: 31779072 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

