Delivery of silver sulfadiazine and adipose derived stem cells using fibrin hydrogel improves infected burn wound regeneration
- PMID: 31194776
- PMCID: PMC6563979
- DOI: 10.1371/journal.pone.0217965
Delivery of silver sulfadiazine and adipose derived stem cells using fibrin hydrogel improves infected burn wound regeneration
Abstract
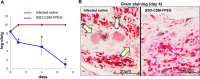
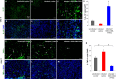
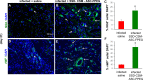
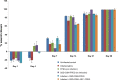
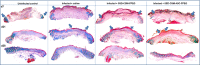
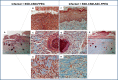
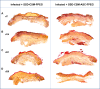
Infection control is necessary for improved burn wound regeneration. In this study contact burn wounds were induced on the dorsum of the rats and were infected with Pseudomonas aeruginosa (107cfu/ml of saline) and left overnight (12-14 hours) to establish the infection. After 12 hours, the wounds were treated with PEGylated fibrin hydrogel containing 50 mgs of silver sulfadiazine (SSD) loaded chitosan microsphere (SSD-CSM-FPEG). On day 9, SSD-CSM-FPEG treated burn wounds further received adipose derived stem cell (5×104 ASCs cells/ml) embedded in PEGylated fibrin hydrogel. Wounds were assessed for the healing outcomes such as neovascularization, granulation tissue formation, wound closure and collagen maturation. Analysis of bacterial load in the burn wound biopsies, demonstrated that SSD-CSM-FPEG significantly reduced bacterial infection, while overt infection was still observed in the untreated groups on day 14. Sequential treatment of infected wounds with SSD-CSM-FPEG followed by ASC-FPEGs (SSD-CSM-ASC-FPEG) significantly reduced bacterial colonization (9 log reduction) and pro-inflammatory cytokine (TNF-α) expression. A significant increase in neovascularization markers; NG2 and vWF was also observed. Histological analysis indicated the wounds treated with SSD-CSM-ASC-FPEG increased amount of dermal collagen matrix deposition, a thicker granulation tissue on day 21 and more mature collagen on day 28. This work demonstrates that the sequential treatment of infected burn wounds with SSD-CSM-FPEG followed by ASC-FPEG reduces bacterial infection as well as promotes neo-vascularization with improved matrix remodeling.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
A PEGylated fibrin hydrogel-based antimicrobial wound dressing controls infection without impeding wound healing.Int Wound J. 2017 Dec;14(6):1248-1257. doi: 10.1111/iwj.12791. Epub 2017 Aug 2. Int Wound J. 2017. PMID: 28771993 Free PMC article.
-
Delivery of Allogeneic Adipose Stem Cells in Polyethylene Glycol-Fibrin Hydrogels as an Adjunct to Meshed Autografts After Sharp Debridement of Deep Partial Thickness Burns.Stem Cells Transl Med. 2018 Apr;7(4):360-372. doi: 10.1002/sctm.17-0160. Epub 2018 Feb 18. Stem Cells Transl Med. 2018. PMID: 29457376 Free PMC article.
-
A PEGylated fibrin-based wound dressing with antimicrobial and angiogenic activity.Acta Biomater. 2011 Jul;7(7):2787-96. doi: 10.1016/j.actbio.2011.04.003. Epub 2011 Apr 13. Acta Biomater. 2011. PMID: 21515420
-
The effects of honey compared to silver sulfadiazine for the treatment of burns: A systematic review of randomized controlled trials.Burns. 2017 Feb;43(1):50-57. doi: 10.1016/j.burns.2016.07.004. Epub 2016 Aug 28. Burns. 2017. PMID: 27576926 Review.
-
The role of silver sulphadiazine in the conservative treatment of partial thickness burn wounds: A systematic review.Burns. 2016 Nov;42(7):1377-1386. doi: 10.1016/j.burns.2016.03.029. Epub 2016 Apr 26. Burns. 2016. PMID: 27126813 Review.
Cited by
-
Evaluation of Fibrin-Agarose Tissue-Like Hydrogels Biocompatibility for Tissue Engineering Applications.Front Bioeng Biotechnol. 2020 Jun 16;8:596. doi: 10.3389/fbioe.2020.00596. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32612984 Free PMC article.
-
Clinical Translational Potential in Skin Wound Regeneration for Adipose-Derived, Blood-Derived, and Cellulose Materials: Cells, Exosomes, and Hydrogels.Biomolecules. 2020 Sep 27;10(10):1373. doi: 10.3390/biom10101373. Biomolecules. 2020. PMID: 32992554 Free PMC article. Review.
-
Polymeric Hydrogel Scaffolds: Skin Tissue Engineering and Regeneration.Adv Pharm Bull. 2022 May;12(3):437-448. doi: 10.34172/apb.2022.069. Epub 2021 Sep 14. Adv Pharm Bull. 2022. PMID: 35935050 Free PMC article. Review.
-
Advancing burn wound treatment: exploring hydrogel as a transdermal drug delivery system.Drug Deliv. 2024 Dec;31(1):2300945. doi: 10.1080/10717544.2023.2300945. Epub 2024 Feb 16. Drug Deliv. 2024. PMID: 38366562 Free PMC article. Review.
-
The efficacy of adipose-derived stem cells in burn injuries: a systematic review.Cell Mol Biol Lett. 2024 Jan 5;29(1):10. doi: 10.1186/s11658-023-00526-w. Cell Mol Biol Lett. 2024. PMID: 38182971 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

