An Oncolytic Adenovirus Vector Expressing p14 FAST Protein Induces Widespread Syncytium Formation and Reduces Tumor Growth Rate In Vivo
- PMID: 31193718
- PMCID: PMC6539411
- DOI: 10.1016/j.omto.2019.05.001
An Oncolytic Adenovirus Vector Expressing p14 FAST Protein Induces Widespread Syncytium Formation and Reduces Tumor Growth Rate In Vivo
Abstract
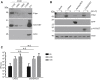
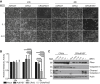
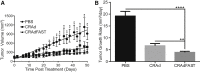
Intratumoral injection of oncolytic viruses provides a direct means of tumor cell destruction for inoperable tumors. Unfortunately, oncolytic vectors based on human adenovirus (HAdV) typically do not spread efficiently throughout the tumor mass, reducing the efficacy of treatment. In this study, we explore the efficacy of a conditionally replicating HAdV vector expressing the p14 Fusion-Associated Small Transmembrane (FAST) protein (CRAdFAST) in both immunocompetent and immunodeficient mouse models of cancer. The p14 FAST protein mediates cell-cell fusion, which may enhance spread of the virus-mediated, tumor cell-killing effect. In the murine 4T1 model of cancer, treatment with CRAdFAST resulted in enhanced cell death compared to vector lacking the p14 FAST gene, but it did not reduce the tumor growth rate in vivo. In the human A549 lung adenocarcinoma model of cancer, CRAdFAST showed significantly improved oncolytic efficacy in vitro and in vivo. In an A549 xenograft tumor model in vivo, CRAdFAST induced tumor cell fusion, which led to the formation of large acellular regions within the tumor and significantly reduced the tumor growth rate compared to control vector. Our results indicate that expression of p14 FAST from an oncolytic HAdV can improve vector efficacy for the treatment of cancer.
Keywords: FAST; adenovirus; cancer; cell fusion; oncolytic.
Figures

Similar articles
-
Adenovirus-Mediated Expression of the p14 Fusion-Associated Small Transmembrane Protein Promotes Cancer Cell Fusion and Apoptosis In Vitro but Does Not Provide Therapeutic Efficacy in a Xenograft Mouse Model of Cancer.PLoS One. 2016 Mar 17;11(3):e0151516. doi: 10.1371/journal.pone.0151516. eCollection 2016. PLoS One. 2016. PMID: 26986751 Free PMC article.
-
Reovirus FAST Protein Enhances Vesicular Stomatitis Virus Oncolytic Virotherapy in Primary and Metastatic Tumor Models.Mol Ther Oncolytics. 2017 Aug 4;6:80-89. doi: 10.1016/j.omto.2017.08.001. eCollection 2017 Sep 15. Mol Ther Oncolytics. 2017. PMID: 28856238 Free PMC article.
-
Expression of the fusogenic p14 FAST protein from a replication-defective adenovirus vector does not provide a therapeutic benefit in an immunocompetent mouse model of cancer.Cancer Gene Ther. 2016 Oct;23(10):355-364. doi: 10.1038/cgt.2016.41. Epub 2016 Oct 14. Cancer Gene Ther. 2016. PMID: 27740615 Free PMC article.
-
The genome position of a therapeutic transgene strongly influences the level of expression in an armed oncolytic human adenovirus vector.Virology. 2021 Sep;561:87-97. doi: 10.1016/j.virol.2021.06.005. Epub 2021 Jun 18. Virology. 2021. PMID: 34171766
-
A capsid-modified, conditionally replicating oncolytic adenovirus vector expressing TRAIL Leads to enhanced cancer cell killing in human glioblastoma models.Cancer Res. 2007 Sep 15;67(18):8783-90. doi: 10.1158/0008-5472.CAN-07-0357. Cancer Res. 2007. PMID: 17875719
Cited by
-
Use of cell fusion proteins to enhance adenoviral vector efficacy as an anti-cancer therapeutic.Cancer Gene Ther. 2021 Aug;28(7-8):745-756. doi: 10.1038/s41417-020-0192-9. Epub 2020 Jul 1. Cancer Gene Ther. 2021. PMID: 32606392 Review.
-
Viruses as tools in gene therapy, vaccine development, and cancer treatment.Arch Virol. 2022 Jun;167(6):1387-1404. doi: 10.1007/s00705-022-05432-8. Epub 2022 Apr 24. Arch Virol. 2022. PMID: 35462594 Free PMC article. Review.
-
Adenovirus Receptor Expression in Cancer and Its Multifaceted Role in Oncolytic Adenovirus Therapy.Int J Mol Sci. 2020 Sep 17;21(18):6828. doi: 10.3390/ijms21186828. Int J Mol Sci. 2020. PMID: 32957644 Free PMC article. Review.
-
Development and application of oncolytic viruses as the nemesis of tumor cells.Front Microbiol. 2023 Jun 12;14:1188526. doi: 10.3389/fmicb.2023.1188526. eCollection 2023. Front Microbiol. 2023. PMID: 37440883 Free PMC article. Review.
-
Fusogenic oncolytic vaccinia virus enhances systemic antitumor immune response by modulating the tumor microenvironment.Mol Ther. 2021 May 5;29(5):1782-1793. doi: 10.1016/j.ymthe.2020.12.024. Epub 2020 Dec 19. Mol Ther. 2021. PMID: 33348052 Free PMC article.
References
-
- Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65:87–108. - PubMed
-
- Stojdl D.F., Lichty B., Knowles S., Marius R., Atkins H., Sonenberg N., Bell J.C. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 2000;6:821–825. - PubMed
-
- Parato K.A., Senger D., Forsyth P.A., Bell J.C. Recent progress in the battle between oncolytic viruses and tumours. Nat. Rev. Cancer. 2005;5:965–976. - PubMed
-
- Lawler S.E., Speranza M.-C., Cho C.-F., Chiocca E.A. Oncolytic viruses in cancer treatment: a review. JAMA Oncol. 2017;3:841–849. - PubMed
-
- DeWeese T.L., van der Poel H., Li S., Mikhak B., Drew R., Goemann M., Hamper U., DeJong R., Detorie N., Rodriguez R. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61:7464–7472. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

