The Best for the Most Important: Maintaining a Pristine Proteome in Stem and Progenitor Cells
- PMID: 31191665
- PMCID: PMC6525796
- DOI: 10.1155/2019/1608787
The Best for the Most Important: Maintaining a Pristine Proteome in Stem and Progenitor Cells
Abstract
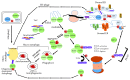
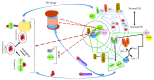
Pluripotent stem cells give rise to reproductively enabled offsprings by generating progressively lineage-restricted multipotent stem cells that would differentiate into lineage-committed stem and progenitor cells. These lineage-committed stem and progenitor cells give rise to all adult tissues and organs. Adult stem and progenitor cells are generated as part of the developmental program and play critical roles in tissue and organ maintenance and/or regeneration. The ability of pluripotent stem cells to self-renew, maintain pluripotency, and differentiate into a multicellular organism is highly dependent on sensing and integrating extracellular and extraorganismal cues. Proteins perform and integrate almost all cellular functions including signal transduction, regulation of gene expression, metabolism, and cell division and death. Therefore, maintenance of an appropriate mix of correctly folded proteins, a pristine proteome, is essential for proper stem cell function. The stem cells' proteome must be pristine because unfolded, misfolded, or otherwise damaged proteins would interfere with unlimited self-renewal, maintenance of pluripotency, differentiation into downstream lineages, and consequently with the development of properly functioning tissue and organs. Understanding how various stem cells generate and maintain a pristine proteome is therefore essential for exploiting their potential in regenerative medicine and possibly for the discovery of novel approaches for maintaining, propagating, and differentiating pluripotent, multipotent, and adult stem cells as well as induced pluripotent stem cells. In this review, we will summarize cellular networks used by various stem cells for generation and maintenance of a pristine proteome. We will also explore the coordination of these networks with one another and their integration with the gene regulatory and signaling networks.
Figures

Similar articles
-
MicroRNA-based system in stem cell reprogramming; differentiation/dedifferentiation.Int J Biochem Cell Biol. 2014 Oct;55:318-28. doi: 10.1016/j.biocel.2014.08.008. Epub 2014 Aug 20. Int J Biochem Cell Biol. 2014. PMID: 25150833 Review.
-
Stem cells and the evolving notion of cellular identity.Philos Trans R Soc Lond B Biol Sci. 2015 Oct 19;370(1680):20140376. doi: 10.1098/rstb.2014.0376. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26416685 Free PMC article. Review.
-
Constraining the Pluripotent Fate of Human Embryonic Stem Cells for Tissue Engineering and Cell Therapy - The Turning Point of Cell-Based Regenerative Medicine.Br Biotechnol J. 2013 Oct 1;3(4):424-457. doi: 10.9734/BBJ/2013/4309#sthash.6D8Rulbv.dpuf. Br Biotechnol J. 2013. PMID: 24926434 Free PMC article.
-
Regulation of Stem Cell Self-Renewal and Oncogenesis by RNA-Binding Proteins.Adv Exp Med Biol. 2016;907:153-88. doi: 10.1007/978-3-319-29073-7_7. Adv Exp Med Biol. 2016. PMID: 27256386
-
Yes-Associated Protein and PDZ Binding Motif: A Critical Signaling Pathway in the Control of Human Pluripotent Stem Cells Self-Renewal and Differentiation.Cell Reprogram. 2020 Apr;22(2):55-61. doi: 10.1089/cell.2019.0084. Epub 2020 Mar 3. Cell Reprogram. 2020. PMID: 32125897 Review.
References
-
- Noormohammadi A., Calculli G., Gutierrez-Garcia R., Khodakarami A., Koyuncu S., Vilchez D. Mechanisms of protein homeostasis (proteostasis) maintain stem cell identity in mammalian pluripotent stem cells. Cellular and Molecular Life Sciences. 2018;75(2):275–290. doi: 10.1007/s00018-017-2602-1. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

