Insulin-like growth factor II mRNA-binding protein 3 promotes cell proliferation, migration and invasion in human glioblastoma
- PMID: 31190868
- PMCID: PMC6527097
- DOI: 10.2147/OTT.S200901
Insulin-like growth factor II mRNA-binding protein 3 promotes cell proliferation, migration and invasion in human glioblastoma
Abstract
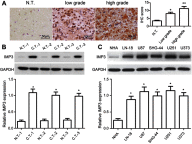
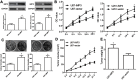
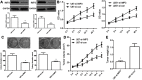
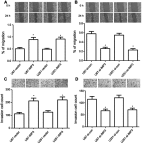
Background/Aims: Recently, the insulin-like growth factor mRNA-binding protein 3 (IMP3) has been reported to be involved in tumorigenesis. We aimed to study the expression and role of IMP3 in human glioblastoma. Methods: We analyzed the expression of IMP3 in 70 cases of glioma tissues, normal brain tissues and 5 kinds of cell lines using western blot. Immunohistochemistry (IHC) was used to evaluate the expression and distribution of IMP3 in glioma tissues. Colony formation, wound healing, migration and invasion assays and tumorigenesis in nude mice were used to explore the function of IMP3 in vitro and in vivo. The epithelial-mesenchymal transition (EMT)-related biomarkers were detected by western blot. Results: We found that the expression level of IMP3 was obviously higher in glioma tissues than that in normal brain tissues, and associated with glioma grade. In-vitro assays revealed that IMP3 overexpression significantly induced cell proliferation, migration, and invasion. Mechanically, IMP3 over-expression downregulated the expression of E-cadherin, but upregulated the expressions of N-cadherin, vimentin, snail, slug and MMP9. However, the inhibition of IMP3 impaired these oncogenic effects. In vivo assay also demonstrated that silencing of IMP3 inhibited tumor growth and improved survival of tumor-bearing xenograft nude mice. Conclusion: IMP3 can promote cell proliferation, migration and invasion by inducing EMT in glioblastoma. Thus, targeting IMP3 pathway may be a novel way to treat patients with glioblastoma.
Keywords: IMP3; glioblastoma; invasion; migration; proliferation.
Conflict of interest statement
The authors declare that they have no conflicts of interest in this work.
Figures

Similar articles
-
IMP3 expression is associated with epithelial-mesenchymal transition in breast cancer.Int J Clin Exp Pathol. 2014 May 15;7(6):3008-17. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25031719 Free PMC article.
-
Triptolide reverses epithelial-mesenchymal transition in glioma cells via inducing autophagy.Ann Transl Med. 2021 Aug;9(16):1304. doi: 10.21037/atm-21-2944. Ann Transl Med. 2021. PMID: 34532441 Free PMC article.
-
The PDK1/c‑Jun pathway activated by TGF‑β induces EMT and promotes proliferation and invasion in human glioblastoma.Int J Oncol. 2018 Nov;53(5):2067-2080. doi: 10.3892/ijo.2018.4525. Epub 2018 Aug 14. Int J Oncol. 2018. PMID: 30106127
-
Insulin-like growth factor II mRNA binding protein 3 regulates proliferation, invasion and migration of neuroendocrine cancer cells.Int J Clin Exp Pathol. 2017 Oct 1;10(10):10269-10275. eCollection 2017. Int J Clin Exp Pathol. 2017. PMID: 31966361 Free PMC article.
-
Insulin-like growth factor II-messenger RNA-binding protein-3 and lung cancer.Biotech Histochem. 2012 Jan;87(1):24-9. doi: 10.3109/10520295.2011.591831. Epub 2011 Aug 15. Biotech Histochem. 2012. PMID: 21838610 Review.
Cited by
-
Insulin-like growth factor II mRNA binding protein 3 is highly expressed in primary diffuse large B-cell lymphoma of the CNS.J Clin Exp Hematop. 2024;64(3):203-207. doi: 10.3960/jslrt.24025. J Clin Exp Hematop. 2024. PMID: 39343609 Free PMC article.
-
Trophectoderm-Specific Knockdown of LIN28 Decreases Expression of Genes Necessary for Cell Proliferation and Reduces Elongation of Sheep Conceptus.Int J Mol Sci. 2020 Apr 6;21(7):2549. doi: 10.3390/ijms21072549. Int J Mol Sci. 2020. PMID: 32268593 Free PMC article.
-
The Critical Role of RNA m6A Methylation in Gliomas: Targeting the Hallmarks of Cancer.Cell Mol Neurobiol. 2023 Jul;43(5):1697-1718. doi: 10.1007/s10571-022-01283-8. Epub 2022 Sep 15. Cell Mol Neurobiol. 2023. PMID: 36104608 Review.
-
N6-methyladenosine reader IGF2BP3 as a prognostic Biomarker contribute to malignant progression of glioma.Transl Cancer Res. 2023 Apr 28;12(4):992-1005. doi: 10.21037/tcr-23-449. Epub 2023 Apr 25. Transl Cancer Res. 2023. PMID: 37180667 Free PMC article.
-
Depletion of the N6-Methyladenosine (m6A) reader protein IGF2BP3 induces ferroptosis in glioma by modulating the expression of GPX4.Cell Death Dis. 2024 Mar 1;15(3):181. doi: 10.1038/s41419-024-06486-z. Cell Death Dis. 2024. PMID: 38429265 Free PMC article.
References
-
- Salloum R, Hummel TR, Kumar SS, et al. A molecular biology and phase II study of imetelstat (GRN163L) in children with recurrent or refractory central nervous system malignancies: a pediatric brain tumor consortium study. J Neurooncol. 2016;129(3):443–451. doi:10.1007/s11060-016-2189-7 - DOI - PMC - PubMed
-
- Wick W, Gorlia T, Bady P, et al. Phase II study of radiotherapy and temsirolimus versus radiochemotherapy with temozolomide in patients with newly diagnosed glioblastoma without MGMT promoter hypermethylation (EORTC 26082). Clin Cancer Res. 2016;22(19):4797–4806. doi:10.1158/1078-0432.CCR-15-3153 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

