Tissue-Specific Gene Expression during Productive Human Papillomavirus 16 Infection of Cervical, Foreskin, and Tonsil Epithelium
- PMID: 31189705
- PMCID: PMC6694821
- DOI: 10.1128/JVI.00915-19
Tissue-Specific Gene Expression during Productive Human Papillomavirus 16 Infection of Cervical, Foreskin, and Tonsil Epithelium
Abstract
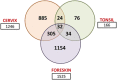
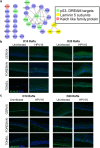
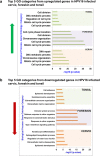
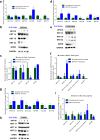
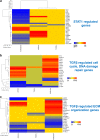
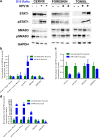
Epidemiological data confirm a much higher incidence of high-risk human papillomavirus 16 (HPV16)-mediated carcinogenesis of the cervical epithelium than for other target sites. In order to elucidate tissue-specific responses to virus infection, we compared gene expression changes induced by productive HPV16 infection of cervical, foreskin, and tonsil organotypic rafts. These rafts closely mimic persistent HPV16 infection, long before carcinogenesis sets in. The total number of gene expression changes varied considerably across the tissue types, with only 32 genes being regulated in common. Among them, we confirmed the Kelch-like family protein KLHL35 and the laminin-5 complex to be upregulated and downregulated, respectively, in all the three tissues. HPV16 infection induces upregulation of genes involved in cell cycle control, cell division, mitosis, DNA replication, and DNA damage repair in all the three tissues, indicative of a hyperproliferative environment. In the cervical and tonsil epithelium, we observe significant downregulation of genes involved in epidermis development, keratinocyte differentiation, and extracellular matrix organization. On the other hand, in HPV16-positive foreskin (HPV16 foreskin) tissue, several genes involved in interferon-mediated innate immunity, cytokine signaling, and cellular defenses were downregulated. Furthermore, pathway analysis and experimental validations identified important cellular pathways like STAT1 and transforming growth factor β (TGF-β) to be differentially regulated among the three tissue types. The differential modulation of important cellular pathways like TGF-β1 and STAT1 can explain the sensitivity of tissues to HPV cancer progression.IMPORTANCE Although the high-risk human papillomavirus 16 infects anogenital and oropharyngeal sites, the cervical epithelium has a unique vulnerability to progression of cancer. Host responses during persistent infection and preneoplastic stages can shape the outcome of cancer progression in a tissue-dependent manner. Our study for the first time reports differential regulation of critical cellular functions and signaling pathways during productive HPV16 infection of cervical, foreskin, and tonsil tissues. While the virus induces hyperproliferation in infected cells, it downregulates epithelial differentiation, epidermal development, and innate immune responses, according to the tissue type. Modulation of these biological functions can determine virus fitness and pathogenesis and illuminate key cellular mechanisms that the virus employs to establish persistence and finally initiate disease progression.
Keywords: HPV16; epithelial differentiation; gene expression; human papillomavirus; immune response; tissue specific.
Copyright © 2019 American Society for Microbiology.
Figures



Similar articles
-
Inhibition of Epstein-Barr Virus Replication in Human Papillomavirus-Immortalized Keratinocytes.J Virol. 2019 Jan 4;93(2):e01216-18. doi: 10.1128/JVI.01216-18. Print 2019 Jan 15. J Virol. 2019. PMID: 30381489 Free PMC article.
-
Microarray analysis of human keratinocytes from different anatomic sites reveals site-specific immune signaling and responses to human papillomavirus type 16 transfection.Mol Med. 2018 May 16;24(1):23. doi: 10.1186/s10020-018-0022-9. Mol Med. 2018. PMID: 30134802 Free PMC article.
-
SAMHD1 Regulates Human Papillomavirus 16-Induced Cell Proliferation and Viral Replication during Differentiation of Keratinocytes.mSphere. 2019 Aug 7;4(4):e00448-19. doi: 10.1128/mSphere.00448-19. mSphere. 2019. PMID: 31391281 Free PMC article.
-
Modelling human papillomavirus biology in oropharyngeal keratinocytes.Philos Trans R Soc Lond B Biol Sci. 2019 May 27;374(1773):20180289. doi: 10.1098/rstb.2018.0289. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 30955493 Free PMC article. Review.
-
HPV: from infection to cancer.Biochem Soc Trans. 2007 Dec;35(Pt 6):1456-60. doi: 10.1042/BST0351456. Biochem Soc Trans. 2007. PMID: 18031245 Review.
Cited by
-
Genome-Wide Transcriptome Analysis of Human Papillomavirus 16-Infected Primary Keratinocytes Reveals Subtle Perturbations Mostly due to E7 Protein Expression.J Virol. 2020 Jan 17;94(3):e01360-19. doi: 10.1128/JVI.01360-19. Print 2020 Jan 17. J Virol. 2020. PMID: 31748387 Free PMC article.
-
The Key Differences between Human Papillomavirus-Positive and -Negative Head and Neck Cancers: Biological and Clinical Implications.Cancers (Basel). 2021 Oct 17;13(20):5206. doi: 10.3390/cancers13205206. Cancers (Basel). 2021. PMID: 34680354 Free PMC article. Review.
-
Generation of human tonsil epithelial organoids as an ex vivo model for SARS-CoV-2 infection.Biomaterials. 2022 Apr;283:121460. doi: 10.1016/j.biomaterials.2022.121460. Epub 2022 Mar 7. Biomaterials. 2022. PMID: 35286852 Free PMC article.
-
The HPV Induced Cancer Resource (THInCR): a Suite of Tools for Investigating HPV-Dependent Human Carcinogenesis.mSphere. 2022 Aug 31;7(4):e0031722. doi: 10.1128/msphere.00317-22. Epub 2022 Aug 11. mSphere. 2022. PMID: 35950764 Free PMC article.
-
Characterization of 3D organotypic epithelial tissues reveals tonsil-specific differences in tonic interferon signaling.bioRxiv [Preprint]. 2023 Jan 20:2023.01.19.524743. doi: 10.1101/2023.01.19.524743. bioRxiv. 2023. Update in: PLoS One. 2023 Oct 4;18(10):e0292368. doi: 10.1371/journal.pone.0292368 PMID: 36711548 Free PMC article. Updated. Preprint.
References
-
- Centers for Disease Control and Prevention. 2012. Human papillomavirus-associated cancers—United States, 2004–2008. MMWR Morb Mortal Wkly Rep 61:258–261. - PubMed
-
- Giuliano AR, Tortolero-Luna G, Ferrer E, Burchell AN, de Sanjose S, Kjaer SK, Munoz N, Schiffman M, Bosch FX. 2008. Epidemiology of human papillomavirus infection in men, cancers other than cervical and benign conditions. Vaccine 26(Suppl 10):K17–K28. doi:10.1016/j.vaccine.2008.06.021. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

