Neutralization of rhesus cytomegalovirus IL-10 reduces horizontal transmission and alters long-term immunity
- PMID: 31189602
- PMCID: PMC6601001
- DOI: 10.1073/pnas.1903317116
Neutralization of rhesus cytomegalovirus IL-10 reduces horizontal transmission and alters long-term immunity
Abstract
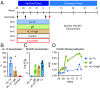
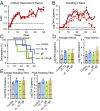
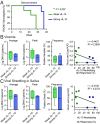
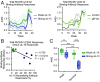
Human cytomegalovirus (HCMV) causes severe disease in infants and immunocompromised people. There is no approved HCMV vaccine, and vaccine development strategies are complicated by evidence of both persistent infection and reinfection of people with prior immunity. The greatest emphasis has been placed on reducing transmission to seronegative pregnant women to prevent vertical transmission and its potentially severe sequelae. Increasing evidence suggests that the earliest host-HCMV interactions establish conditions for viral persistence, including evasion of host immune responses to the virus. Using a nonhuman primate model of HCMV infection, we show that rhesus macaques immunized against viral interleukin-10 (IL-10) manifest delayed rhesus cytomegalovirus (RhCMV) acquisition and altered immune responses to the infection when it does occur. Among animals with the greatest antiviral IL-10-neutralizing activity, the timing of RhCMV seroconversion was delayed by an average of 12 weeks. After acquisition, such animals displayed an antibody response to the new infection, which peaked as expected after 2 weeks but then declined rapidly. In contrast, surprisingly, vaccination with glycoprotein B (gB) protein had no discernible impact on these outcomes. Our results demonstrate that viral IL-10 is a key regulator of successful host immune responses to RhCMV. Viral IL-10 is, therefore, an important target for vaccine strategies against cytomegalovirus (CMV). Furthermore, given the immunoregulatory function of viral IL-10, targeting this protein may prove synergistic with other vaccine therapies and targets. Our study also provides additional evidence that the earliest host-CMV interactions can have a significant impact on the nature of persistent infection.
Keywords: IL-10; cytomegalovirus; rhesus cytomegalovirus; vaccine; viral IL-10.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Pathogenesis of Wild-Type-Like Rhesus Cytomegalovirus Strains following Oral Exposure of Immune-Competent Rhesus Macaques.J Virol. 2022 Feb 9;96(3):e0165321. doi: 10.1128/JVI.01653-21. Epub 2021 Nov 17. J Virol. 2022. PMID: 34788083 Free PMC article.
-
Host immune responses to a viral immune modulating protein: immunogenicity of viral interleukin-10 in rhesus cytomegalovirus-infected rhesus macaques.PLoS One. 2012;7(5):e37931. doi: 10.1371/journal.pone.0037931. Epub 2012 May 24. PLoS One. 2012. PMID: 22655082 Free PMC article.
-
Exploitation of Interleukin-10 (IL-10) Signaling Pathways: Alternate Roles of Viral and Cellular IL-10 in Rhesus Cytomegalovirus Infection.J Virol. 2016 Oct 14;90(21):9920-9930. doi: 10.1128/JVI.00635-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27558431 Free PMC article.
-
Animal Models of Congenital Cytomegalovirus Transmission: Implications for Vaccine Development.J Infect Dis. 2020 Mar 5;221(Suppl 1):S60-S73. doi: 10.1093/infdis/jiz484. J Infect Dis. 2020. PMID: 32134481 Free PMC article. Review.
-
Prospects of a vaccine for the prevention of congenital cytomegalovirus disease.Med Microbiol Immunol. 2016 Dec;205(6):537-547. doi: 10.1007/s00430-016-0472-z. Epub 2016 Aug 12. Med Microbiol Immunol. 2016. PMID: 27519596 Review.
Cited by
-
Human Cytomegalovirus Infection in Women With Preexisting Immunity: Sources of Infection and Mechanisms of Infection in the Presence of Antiviral Immunity.J Infect Dis. 2020 Mar 5;221(Suppl 1):S1-S8. doi: 10.1093/infdis/jiz464. J Infect Dis. 2020. PMID: 32134479 Free PMC article. Review.
-
A Review on Zoonotic Pathogens Associated with Non-Human Primates: Understanding the Potential Threats to Humans.Microorganisms. 2023 Jan 18;11(2):246. doi: 10.3390/microorganisms11020246. Microorganisms. 2023. PMID: 36838210 Free PMC article. Review.
-
MVA-Vectored Pentameric Complex (PC) and gB Vaccines Improve Pregnancy Outcome after Guinea Pig CMV Challenge, but Only gB Vaccine Reduces Vertical Transmission.Vaccines (Basel). 2019 Nov 14;7(4):182. doi: 10.3390/vaccines7040182. Vaccines (Basel). 2019. PMID: 31739399 Free PMC article.
-
Modeling Human Cytomegalovirus in Humanized Mice for Vaccine Testing.Vaccines (Basel). 2020 Feb 17;8(1):89. doi: 10.3390/vaccines8010089. Vaccines (Basel). 2020. PMID: 32079250 Free PMC article. Review.
-
Cytokine levels in breast cancer are highly dependent on cytomegalovirus (CMV) status.Breast Cancer Res Treat. 2024 Dec;208(3):631-641. doi: 10.1007/s10549-024-07459-8. Epub 2024 Aug 22. Breast Cancer Res Treat. 2024. PMID: 39172306 Free PMC article.
References
-
- Arvin A. M., Fast P., Myers M., Plotkin S., Rabinovich R.; National Vaccine Advisory Committee , Vaccine development to prevent cytomegalovirus disease: Report from the National Vaccine Advisory Committee. Clin. Infect. Dis. 39, 233–239 (2004). - PubMed
-
- Griffiths P. D., Burden of disease associated with human cytomegalovirus and prospects for elimination by universal immunisation. Lancet Infect. Dis. 12, 790–798 (2012). - PubMed

