Chondrocyte-Specific RUNX2 Overexpression Accelerates Post-traumatic Osteoarthritis Progression in Adult Mice
- PMID: 31189030
- PMCID: PMC7047611
- DOI: 10.1002/jbmr.3737
Chondrocyte-Specific RUNX2 Overexpression Accelerates Post-traumatic Osteoarthritis Progression in Adult Mice
Abstract
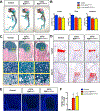
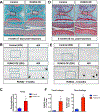
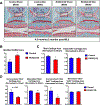
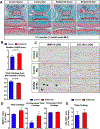
RUNX2 is a transcription factor critical for chondrocyte maturation and normal endochondral bone formation. It promotes the expression of factors catabolic to the cartilage extracellular matrix and is upregulated in human osteoarthritic cartilage and in murine articular cartilage following joint injury. To date, in vivo studies of RUNX2 overexpression in cartilage have been limited to forced expression in osteochondroprogenitor cells preventing investigation into the effects of chondrocyte-specific RUNX2 overexpression in postnatal articular cartilage. Here, we used the Rosa26Runx2 allele in combination with the inducible Col2a1CreERT2 transgene or the inducible AcanCreERT2 knock-in allele to achieve chondrocyte-specific RUNX2 overexpression (OE) during embryonic development or in the articular cartilage of adult mice, respectively. RUNX2 OE was induced at embryonic day 13.5 (E13.5) for all developmental studies. Histology and in situ hybridization analyses suggest an early onset of chondrocyte hypertrophy and accelerated terminal maturation in the limbs of the RUNX2 OE embryos compared to control embryos. For all postnatal studies, RUNX2 OE was induced at 2 months of age. Surprisingly, no histopathological signs of cartilage degeneration were observed even 6 months following induction of RUNX2 OE. Using the meniscal/ligamentous injury (MLI), a surgical model of knee joint destabilization and meniscal injury, however, we found that RUNX2 OE accelerates the progression of cartilage degeneration following joint trauma. One month following MLI, the numbers of MMP13-positive and TUNEL-positive chondrocytes were significantly greater in the articular cartilage of the RUNX2 OE joints compared to control joints and 2 months following MLI, histomorphometry and Osteoarthritis Research Society International (OARSI) scoring revealed decreased cartilage area in the RUNX2 OE joints. Collectively, these results suggest that although RUNX2 overexpression alone may not be sufficient to initiate the OA degenerative process, it may predetermine the rate of OA onset and/or progression following traumatic joint injury. © 2019 American Society for Bone and Mineral Research.
Keywords: ANIMAL MODELS; CELL/TISSUE SIGNALING; CHONDROCYTE AND CARTILAGE BIOLOGY; DISEASES AND DISORDERS OF/RELATED TO BONE; GENETIC ANIMAL MODELS; HYPERTROPHY; OSTEOARTHRITIS; POST-TRAUMATIC OSTEOARTHRITIS; RUNX2; TRANSCRIPTION FACTORS.
© 2019 American Society for Bone and Mineral Research.
Figures

Similar articles
-
Deletion of Runx2 in Articular Chondrocytes Decelerates the Progression of DMM-Induced Osteoarthritis in Adult Mice.Sci Rep. 2017 May 24;7(1):2371. doi: 10.1038/s41598-017-02490-w. Sci Rep. 2017. PMID: 28539595 Free PMC article.
-
Bushenhuoxue formula attenuates cartilage degeneration in an osteoarthritic mouse model through TGF-β/MMP13 signaling.J Transl Med. 2018 Mar 20;16(1):72. doi: 10.1186/s12967-018-1437-3. J Transl Med. 2018. PMID: 29554973 Free PMC article.
-
Teriparatide as a chondroregenerative therapy for injury-induced osteoarthritis.Sci Transl Med. 2011 Sep 21;3(101):101ra93. doi: 10.1126/scitranslmed.3002214. Sci Transl Med. 2011. PMID: 21937758 Free PMC article.
-
Endochondral ossification signals in cartilage degradation during osteoarthritis progression in experimental mouse models.Mol Cells. 2008 Feb 29;25(1):1-6. Mol Cells. 2008. PMID: 18319608 Review.
-
Molecular regulation of articular chondrocyte function and its significance in osteoarthritis.Histol Histopathol. 2011 Mar;26(3):377-94. doi: 10.14670/HH-26.377. Histol Histopathol. 2011. PMID: 21210351 Review.
Cited by
-
Regulation of Skeletal Development and Maintenance by Runx2 and Sp7.Int J Mol Sci. 2024 Sep 20;25(18):10102. doi: 10.3390/ijms251810102. Int J Mol Sci. 2024. PMID: 39337587 Free PMC article. Review.
-
Palovarotene Action Against Heterotopic Ossification Includes a Reduction of Local Participating Activin A-Expressing Cell Populations.JBMR Plus. 2023 Oct 19;7(12):e10821. doi: 10.1002/jbm4.10821. eCollection 2023 Dec. JBMR Plus. 2023. PMID: 38130748 Free PMC article.
-
Molecular Mechanism of Runx2-Dependent Bone Development.Mol Cells. 2020 Feb 29;43(2):168-175. doi: 10.14348/molcells.2019.0244. Mol Cells. 2020. PMID: 31896233 Free PMC article. Review.
-
Heterozygous LRP1 deficiency causes developmental dysplasia of the hip by impairing triradiate chondrocytes differentiation due to inhibition of autophagy.Proc Natl Acad Sci U S A. 2022 Sep 13;119(37):e2203557119. doi: 10.1073/pnas.2203557119. Epub 2022 Sep 6. Proc Natl Acad Sci U S A. 2022. PMID: 36067312 Free PMC article.
-
Transient neonatal shoulder paralysis causes early osteoarthritis in a mouse model.J Orthop Res. 2022 Sep;40(9):1981-1992. doi: 10.1002/jor.25225. Epub 2021 Dec 3. J Orthop Res. 2022. PMID: 34812543 Free PMC article.
References
-
- Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. November 1 2002;16(21):2813–28. Epub 2002/11/05. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

