Potent Enhancement of HIV-1 Replication by Nef in the Absence of SERINC3 and SERINC5
- PMID: 31186327
- PMCID: PMC6561029
- DOI: 10.1128/mBio.01071-19
Potent Enhancement of HIV-1 Replication by Nef in the Absence of SERINC3 and SERINC5
Abstract
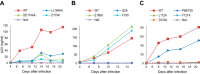
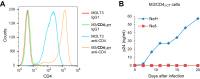
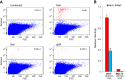
It has recently emerged that HIV-1 Nef counteracts the antiviral host proteins SERINC3 and SERINC5. In particular, SERINC5 inhibits the infectivity of progeny virions when incorporated. SERINC3 and SERINC5 are also counteracted by the unrelated murine leukemia virus glycosylated Gag (glycoGag) protein, which possesses a potent Nef-like activity on HIV-1 infectivity. We now report that a minimal glycoGag termed glycoMA can fully substitute for Nef in promoting HIV-1 replication in Jurkat T lymphoid cells, indicating that Nef enhances replication in these cells mainly by counteracting SERINCs. In contrast, the SERINC antagonist glycoMA was unable to substitute for Nef in MOLT-3 T lymphoid cells, in which HIV-1 replication was highly dependent on Nef, and remained so even in the absence of SERINC3 and SERINC5. As in MOLT-3 cells, glycoMA was unable to substitute for Nef in stimulating HIV-1 replication in primary human cells. Although the ability of Nef mutants to promote HIV-1 replication in MOLT-3 cells correlated with the ability to engage endocytic machinery and to downregulate CD4, Nef nevertheless rescued virus replication under conditions where CD4 downregulation did not occur. Taken together, our observations raise the possibility that Nef triggers the endocytosis of a novel antiviral factor that is active against both laboratory-adapted and primary HIV-1 strains.IMPORTANCE The Nef protein of HIV-1 and the unrelated glycoGag protein of a murine leukemia virus similarly prevent the uptake of antiviral host proteins called SERINC3 and SERINC5 into HIV-1 particles, which enhances their infectiousness. We now show that although both SERINC antagonists can in principle similarly enhance HIV-1 replication, glycoGag is unable to substitute for Nef in primary human cells and in a T cell line called MOLT-3. In MOLT-3 cells, Nef remained crucial for HIV-1 replication even in the absence of SERINC3 and SERINC5. The pronounced effect of Nef on HIV-1 spreading in MOLT-3 cells correlated with the ability of Nef to engage cellular endocytic machinery and to downregulate the HIV-1 receptor CD4 but nevertheless persisted in the absence of CD4 downregulation. Collectively, our results provide evidence for a potent novel restriction activity that affects even relatively SERINC-resistant HIV-1 isolates and is counteracted by Nef.
Keywords: Nef; SERINC5; human immunodeficiency virus; infectivity; virus replication.
Copyright © 2019 Wu et al.
Figures





Similar articles
-
AP-2 Adaptor Complex-Dependent Enhancement of HIV-1 Replication by Nef in the Absence of the Nef/AP-2 Targets SERINC5 and CD4.mBio. 2023 Feb 28;14(1):e0338222. doi: 10.1128/mbio.03382-22. Epub 2023 Jan 9. mBio. 2023. PMID: 36622146 Free PMC article.
-
SERINC3 and SERINC5 restrict HIV-1 infectivity and are counteracted by Nef.Nature. 2015 Oct 8;526(7572):218-23. doi: 10.1038/nature15400. Epub 2015 Sep 30. Nature. 2015. PMID: 26416733 Free PMC article.
-
Murine Leukemia Virus Glycosylated Gag Reduces Murine SERINC5 Protein Expression at Steady-State Levels via the Endosome/Lysosome Pathway to Counteract SERINC5 Antiretroviral Activity.J Virol. 2019 Jan 4;93(2):e01651-18. doi: 10.1128/JVI.01651-18. Print 2019 Jan 15. J Virol. 2019. PMID: 30355687 Free PMC article.
-
SERINC as a Restriction Factor to Inhibit Viral Infectivity and the Interaction with HIV.J Immunol Res. 2017;2017:1548905. doi: 10.1155/2017/1548905. Epub 2017 Nov 22. J Immunol Res. 2017. PMID: 29359168 Free PMC article. Review.
-
SERINC5 as a New Restriction Factor for Human Immunodeficiency Virus and Murine Leukemia Virus.Annu Rev Virol. 2018 Sep 29;5(1):323-340. doi: 10.1146/annurev-virology-092917-043308. Annu Rev Virol. 2018. PMID: 30265629 Review.
Cited by
-
Nef enhances HIV-1 replication and infectivity independently of SERINC5 in CEM T cells.Virology. 2023 Jan;578:154-162. doi: 10.1016/j.virol.2022.12.008. Epub 2022 Dec 23. Virology. 2023. PMID: 36577173 Free PMC article.
-
Replication competent HIV-guided CRISPR screen identifies antiviral factors including targets of the accessory protein Nef.Nat Commun. 2024 May 7;15(1):3813. doi: 10.1038/s41467-024-48228-x. Nat Commun. 2024. PMID: 38714682 Free PMC article.
-
Natural HIV-1 Nef Polymorphisms Impair SERINC5 Downregulation Activity.Cell Rep. 2019 Nov 5;29(6):1449-1457.e5. doi: 10.1016/j.celrep.2019.10.007. Cell Rep. 2019. PMID: 31693887 Free PMC article.
-
SERINC5 Inhibits the Secretion of Complete and Genome-Free Hepatitis B Virions Through Interfering With the Glycosylation of the HBV Envelope.Front Microbiol. 2020 Apr 30;11:697. doi: 10.3389/fmicb.2020.00697. eCollection 2020. Front Microbiol. 2020. PMID: 32431673 Free PMC article.
-
Aromatic Side Chain at Position 412 of SERINC5 Exerts Restriction Activity toward HIV-1 and Other Retroviruses.J Virol. 2021 Aug 25;95(18):e0063421. doi: 10.1128/JVI.00634-21. Epub 2021 Aug 25. J Virol. 2021. PMID: 34190600 Free PMC article.
References
-
- Deacon NJ, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker DJ, McPhee DA, Greenway AL, Ellett A, Chatfield C, Lawson VA, Crowe S, Maerz A, Sonza S, Learmont J, Sullivan JS, Cunningham A, Dwyer D, Dowton D, Mills J. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988–991. doi:10.1126/science.270.5238.988. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
