Dihydrotestosterone Increases Cytotoxic Activity of Macrophages on Prostate Cancer Cells via TRAIL
- PMID: 31184711
- PMCID: PMC6691685
- DOI: 10.1210/en.2019-00367
Dihydrotestosterone Increases Cytotoxic Activity of Macrophages on Prostate Cancer Cells via TRAIL
Abstract
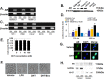
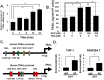
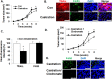
Although androgen deprivation therapy (ADT) and immunotherapy are potential treatment options in men with metastatic prostate cancer (CaP), androgen has conventionally been proposed to be a suppressor of the immune response. However, we herein report that DHT activates macrophages. When the murine macrophage cell line (RAW 264.7), human monocyte cell line (THP-1), and human peripheral blood monocytes were cultured with androgen-resistant CaP cell lines, DHT increased cytotoxicity of macrophages in a concentration-dependent manner. Further studies revealed that DHT induced M1 polarization and increased the expression levels of TNF-related apoptosis-inducing ligand (TRAIL) in macrophages and that this effect was abrogated when TRAIL was neutralized with a blocking antibody or small interfering RNA. Subsequent experiments demonstrated that induction of TRAIL expression was regulated by direct binding of androgen receptor to the TRAIL promoter region. Finally, an in vivo mouse study demonstrated that castration enhanced the growth of an androgen-resistant murine CaP tumor and that this protumorigenic effect of castration was blocked when macrophages were removed with clodronate liposomes. Collectively, these results demonstrate that DHT activates the cytotoxic activity of macrophages and suggest that immunotherapy may not be optimal when combined with ADT in CaP.
Copyright © 2019 Endocrine Society.
Figures


Similar articles
-
Bone morphogenetic protein-6 induces castration resistance in prostate cancer cells through tumor infiltrating macrophages.Cancer Sci. 2013 Aug;104(8):1027-32. doi: 10.1111/cas.12206. Epub 2013 Jun 28. Cancer Sci. 2013. PMID: 23710822 Free PMC article.
-
Cyproterone acetate enhances TRAIL-induced androgen-independent prostate cancer cell apoptosis via up-regulation of death receptor 5.BMC Cancer. 2017 Mar 7;17(1):179. doi: 10.1186/s12885-017-3153-4. BMC Cancer. 2017. PMID: 28270124 Free PMC article.
-
Androgen-dependent cell cycle arrest and apoptotic death in PC-3 prostatic cell cultures expressing a full-length human androgen receptor.Mol Cell Endocrinol. 1997 Jan 3;126(1):59-73. doi: 10.1016/s0303-7207(96)03970-6. Mol Cell Endocrinol. 1997. PMID: 9027364
-
Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence.Cancer Res. 2004 Oct 1;64(19):7156-68. doi: 10.1158/0008-5472.CAN-04-1121. Cancer Res. 2004. PMID: 15466214
-
Concept and viability of androgen annihilation for advanced prostate cancer.Cancer. 2014 Sep 1;120(17):2628-37. doi: 10.1002/cncr.28675. Epub 2014 Apr 25. Cancer. 2014. PMID: 24771515 Free PMC article. Review.
Cited by
-
Effects of 5α-dihydrotestosterone on the modulation of monocyte/macrophage response to Staphylococcus aureus: an in vitro study.Biol Sex Differ. 2023 Mar 31;14(1):15. doi: 10.1186/s13293-023-00501-2. Biol Sex Differ. 2023. PMID: 37004108 Free PMC article.
-
LTF Regulates the Immune Microenvironment of Prostate Cancer Through JAK/STAT3 Pathway.Front Oncol. 2021 Nov 10;11:692117. doi: 10.3389/fonc.2021.692117. eCollection 2021. Front Oncol. 2021. PMID: 34868909 Free PMC article.
-
Sex Steroids Effects on Asthma: A Network Perspective of Immune and Airway Cells.Cells. 2022 Jul 19;11(14):2238. doi: 10.3390/cells11142238. Cells. 2022. PMID: 35883681 Free PMC article. Review.
-
Androgen and Androgen Receptors as Regulators of Monocyte and Macrophage Biology in the Healthy and Diseased Lung.Front Immunol. 2020 Aug 7;11:1698. doi: 10.3389/fimmu.2020.01698. eCollection 2020. Front Immunol. 2020. PMID: 32849595 Free PMC article. Review.
-
RISING STARS: Androgens and immune cell function.J Endocrinol. 2024 Apr 29;261(3):e230398. doi: 10.1530/JOE-23-0398. Print 2024 Jun 1. J Endocrinol. 2024. PMID: 38579776 Free PMC article. Review.
References
-
- Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, Goodwin RG Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3(6):673–682. - PubMed
-
- Hesry V, Piquet-Pellorce C, Travert M, Donaghy L, Jégou B, Patard JJ, Guillaudeux T. Sensitivity of prostate cells to TRAIL-induced apoptosis increases with tumor progression: DR5 and caspase 8 are key players. Prostate. 2006;66(9):987–995. - PubMed
-
- Nakajima Y, DelliPizzi AM, Mallouh C, Ferreri NR. TNF-mediated cytotoxicity and resistance in human prostate cancer cell lines. Prostate. 1996;29(5):296–302. - PubMed
-
- Koschny R, Walczak H, Ganten TM. The promise of TRAIL--potential and risks of a novel anticancer therapy. J Mol Med (Berl). 2007;85(9):923–935. - PubMed
-
- Mahalingam D, Szegezdi E, Keane M, de Jong S, Samali A. TRAIL receptor signalling and modulation: are we on the right TRAIL? Cancer Treat Rev. 2009;35(3):280–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

