The Evolving Role of CD8+CD28- Immunosenescent T Cells in Cancer Immunology
- PMID: 31181772
- PMCID: PMC6600236
- DOI: 10.3390/ijms20112810
The Evolving Role of CD8+CD28- Immunosenescent T Cells in Cancer Immunology
Abstract
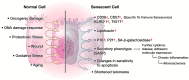
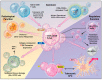
Functional, tumor-specific CD8+ cytotoxic T lymphocytes drive the adaptive immune response to cancer. Thus, induction of their activity is the ultimate aim of all immunotherapies. Success of anti-tumor immunotherapy is precluded by marked immunosuppression in the tumor microenvironment (TME) leading to CD8+ effector T cell dysfunction. Among the many facets of CD8+ T cell dysfunction that have been recognized-tolerance, anergy, exhaustion, and senescence-CD8+ T cell senescence is incompletely understood. Naïve CD8+ T cells require three essential signals for activation, differentiation, and survival through T-cell receptor, costimulatory receptors, and cytokine receptors. Downregulation of costimulatory molecule CD28 is a hallmark of senescent T cells and increased CD8+CD28- senescent populations with heterogeneous roles have been observed in multiple solid and hematogenous tumors. T cell senescence can be induced by several factors including aging, telomere damage, tumor-associated stress, and regulatory T (Treg) cells. Tumor-induced T cell senescence is yet another mechanism that enables tumor cell resistance to immunotherapy. In this paper, we provide a comprehensive overview of CD8+CD28- senescent T cell population, their origin, their function in immunology and pathologic conditions, including TME and their implication for immunotherapy. Further characterization and investigation into this subset of CD8+ T cells could improve the efficacy of future anti-tumor immunotherapy.
Keywords: CD8+CD28− T cells; cancer; cancer immunology; glioblastoma; immunotherapy; malignant glioma.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Aging- and Tumor-Mediated Increase in CD8+CD28- T Cells Might Impose a Strong Barrier to Success of Immunotherapy in Glioblastoma.Immunohorizons. 2021 Jun 8;5(6):395-409. doi: 10.4049/immunohorizons.2100008. Immunohorizons. 2021. PMID: 34103370 Free PMC article.
-
PD1-CD28 Fusion Protein Enables CD4+ T Cell Help for Adoptive T Cell Therapy in Models of Pancreatic Cancer and Non-hodgkin Lymphoma.Front Immunol. 2018 Aug 30;9:1955. doi: 10.3389/fimmu.2018.01955. eCollection 2018. Front Immunol. 2018. PMID: 30214445 Free PMC article.
-
4-1BB is superior to CD28 costimulation for generating CD8+ cytotoxic lymphocytes for adoptive immunotherapy.J Immunol. 2007 Oct 1;179(7):4910-8. doi: 10.4049/jimmunol.179.7.4910. J Immunol. 2007. PMID: 17878391 Free PMC article.
-
Immunosenescence: a key player in cancer development.J Hematol Oncol. 2020 Nov 10;13(1):151. doi: 10.1186/s13045-020-00986-z. J Hematol Oncol. 2020. PMID: 33168037 Free PMC article. Review.
-
The Emerging Role of CD8+ Tissue Resident Memory T (TRM) Cells in Antitumor Immunity: A Unique Functional Contribution of the CD103 Integrin.Front Immunol. 2018 Aug 15;9:1904. doi: 10.3389/fimmu.2018.01904. eCollection 2018. Front Immunol. 2018. PMID: 30158938 Free PMC article. Review.
Cited by
-
Socioeconomic status and immune aging in older US adults in the health and retirement study.Biodemography Soc Biol. 2022 Jul-Dec;67(3-4):187-202. doi: 10.1080/19485565.2022.2149465. Epub 2022 Dec 6. Biodemography Soc Biol. 2022. PMID: 36472376 Free PMC article.
-
p16INK4a Regulates Cellular Senescence in PD-1-Expressing Human T Cells.Front Immunol. 2021 Aug 9;12:698565. doi: 10.3389/fimmu.2021.698565. eCollection 2021. Front Immunol. 2021. PMID: 34434190 Free PMC article.
-
Age-dependent immune profile in healthy individuals: an original study, systematic review and meta-analysis.Immun Ageing. 2024 Oct 29;21(1):75. doi: 10.1186/s12979-024-00480-x. Immun Ageing. 2024. PMID: 39472926 Free PMC article.
-
Exercise and the immune system: taking steps to improve responses to cancer immunotherapy.J Immunother Cancer. 2021 Jul;9(7):e001872. doi: 10.1136/jitc-2020-001872. J Immunother Cancer. 2021. PMID: 34215686 Free PMC article. Review.
-
Pathway-based stratification of gliomas uncovers four subtypes with different TME characteristics and prognosis.J Cell Mol Med. 2024 Apr;28(8):e18208. doi: 10.1111/jcmm.18208. J Cell Mol Med. 2024. PMID: 38613347 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

